Structural insights and shedding light on preferential interactions of dietary flavonoids with G-quadruplex DNA structures: A new horizon
- PMID: 36879969
- PMCID: PMC9984854
- DOI: 10.1016/j.heliyon.2023.e13959
Structural insights and shedding light on preferential interactions of dietary flavonoids with G-quadruplex DNA structures: A new horizon
Abstract
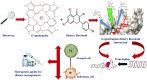
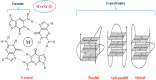
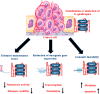
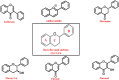
G-quadruplex, a structurally unique structure in nucleic acids present all throughout the human genome, has sparked great attention in therapeutic investigations. Targeting G-quadruplex structure is a new strategy for the drug development. Flavonoids are found in almost all dietary plant-based beverages and food products; therefore, they are ingested in significant proportions through the human diet. Although synthetically developed drug molecules are used vigorously but they have various adverse effects. While on the other hand, nature supplies chemically unique scaffolds in the form of distinct dietary flavonoids that are easily accessible, less poisonous, and have higher bioavailability. Because of their great pharmacological effectiveness and minimal cytotoxicity, such low molecular weight compounds are feasible alternatives to synthetic therapeutic medicines. Therefore, from a drug-development point of view, investigation on screening the binding capabilities of quadruplex-interactive small natural compounds like dietary flavonoids are expected to be highly effective, with a particular emphasis on the selectivity towards polymorphic G-quadruplex structures. In this respect, quadruplexes have scintillated research into their potential interaction with these dietary flavonoids. The purpose of this review is to offer an up-to-date close-up look at the research on their interaction with structurally varied dietary flavonoids with the goal of providing newer perspectives to construct novel therapeutic agents for next-generation disease managements.
Keywords: Dietary flavonoids; G-quadruplex; G-quadruplex ligands; G-quadruplex-DNA probes; G-quadruplex-dietary flavonoids interaction.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
DNA G-Quadruplex in Human Telomeres and Oncogene Promoters: Structures, Functions, and Small Molecule Targeting.Acc Chem Res. 2022 Sep 20;55(18):2628-2646. doi: 10.1021/acs.accounts.2c00337. Epub 2022 Sep 2. Acc Chem Res. 2022. PMID: 36054116 Free PMC article.
-
Exploring the Interactions of the Dietary Plant Flavonoids Fisetin and Naringenin with G-Quadruplex and Duplex DNA, Showing Contrasting Binding Behavior: Spectroscopic and Molecular Modeling Approaches.J Phys Chem B. 2016 Sep 1;120(34):8942-52. doi: 10.1021/acs.jpcb.6b06357. Epub 2016 Aug 16. J Phys Chem B. 2016. PMID: 27491376
-
Developing Novel G-Quadruplex Ligands: from Interaction with Nucleic Acids to Interfering with Nucleic Acid⁻Protein Interaction.Molecules. 2019 Jan 22;24(3):396. doi: 10.3390/molecules24030396. Molecules. 2019. PMID: 30678288 Free PMC article. Review.
-
Enhanced G-quadruplex selectivity of flavonoid glycoside rutin over quercetin.Nat Prod Res. 2022 Jul;36(13):3469-3473. doi: 10.1080/14786419.2020.1859505. Epub 2020 Dec 12. Nat Prod Res. 2022. PMID: 33307807
-
Ligand Design to Acquire Specificity to Intended G-Quadruplex Structures.Chemistry. 2019 Jan 7;25(2):417-430. doi: 10.1002/chem.201802691. Epub 2018 Nov 9. Chemistry. 2019. PMID: 30051593 Review.
Cited by
-
Modulation of the HRAS1 I‑motif DNA Promoter Region by Dietary Plant Flavonoid Kaempferol.ACS Omega. 2025 Jul 18;10(29):31381-31392. doi: 10.1021/acsomega.5c00361. eCollection 2025 Jul 29. ACS Omega. 2025. PMID: 40757292 Free PMC article.
-
Prolidase-proline oxidase axis is engaged in apoptosis induction by birch buds flavonol santin in endometrial adenocarcinoma cell line.Front Mol Biosci. 2023 Sep 6;10:1247536. doi: 10.3389/fmolb.2023.1247536. eCollection 2023. Front Mol Biosci. 2023. PMID: 37745688 Free PMC article.
-
Binding of Quercetin Derivatives toward G-Tetrads as Studied by the Survival Yield Method.ACS Omega. 2023 Oct 11;8(42):39816-39821. doi: 10.1021/acsomega.3c06016. eCollection 2023 Oct 24. ACS Omega. 2023. PMID: 37901583 Free PMC article.
-
Non-canonical DNA structures in the human ribosomal DNA.Histochem Cell Biol. 2023 Dec;160(6):499-515. doi: 10.1007/s00418-023-02233-1. Epub 2023 Sep 26. Histochem Cell Biol. 2023. PMID: 37750997 Review.
-
Uncovering the Contrasting Binding Behavior of Plant Flavonoids Fisetin and Morin Having Subsidiary Hydroxyl Groups (-OH) with HRAS1 and HRAS2 i-Motif DNA Structures: Decoding the Structural Alterations and Positional Influences.ACS Omega. 2023 Aug 7;8(33):30315-30329. doi: 10.1021/acsomega.3c03105. eCollection 2023 Aug 22. ACS Omega. 2023. PMID: 37636929 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

