A novel nano-iron supplement versus standard treatment for iron deficiency anaemia in children 6-35 months (IHAT-GUT trial): a double-blind, randomised, placebo-controlled non-inferiority phase II trial in The Gambia
- PMID: 36880049
- PMCID: PMC9985047
- DOI: 10.1016/j.eclinm.2023.101853
A novel nano-iron supplement versus standard treatment for iron deficiency anaemia in children 6-35 months (IHAT-GUT trial): a double-blind, randomised, placebo-controlled non-inferiority phase II trial in The Gambia
Abstract
Background: Iron deficiency anaemia (IDA) is the leading cause of years lost to disability in most sub-Saharan African countries and is especially common in young children. The IHAT-GUT trial assessed the efficacy and safety of a novel nano iron supplement, which is a dietary ferritin analogue termed iron hydroxide adipate tartrate (IHAT), for the treatment of IDA in children under 3 years of age.
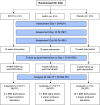
Methods: In this single-country, randomised, double-blind, parallel, placebo-controlled, non-inferiority Phase II study in The Gambia, children 6-35 months with IDA (7≤Hb < 11 g/dL and ferritin<30 μg/L) were randomly assigned (1:1:1) to receive either IHAT, ferrous sulphate (FeSO4) or placebo daily for 3 months (85 days). The daily iron dose was 12.5 mg Fe equivalent for FeSO4 and the estimated dose with comparable iron-bioavailability for IHAT (20 mg Fe). The primary efficacy endpoint was the composite of haemoglobin response at day 85 and correction of iron deficiency. The non-inferiority margin was 0.1 absolute difference in response probability. The primary safety endpoint was moderate-severe diarrhoea analysed as incidence density and prevalence over the 3 months intervention. Secondary endpoints reported herein include hospitalisation, acute respiratory infection, malaria, treatment failures, iron handling markers, inflammatory markers, longitudinal prevalence of diarrhoea and incidence density of bloody diarrhoea. Main analyses were per-protocol (PP) and intention-to-treat (ITT) analyses. This trial is registered with clinicaltrials.gov (NCT02941081).
Findings: Between Nov 2017 and Nov 2018, 642 children were randomised into the study (214 per group) and included in the ITT analysis, the PP population included 582 children. A total of 50/177 (28.2%) children in the IHAT group achieved the primary efficacy endpoint, as compared with 42/190 (22.1%) in the FeSO4 group (OR 1.39, 80% CI 1.01-1.91, PP population) and with 2/186 (1.1%) in the placebo group. Diarrhoea prevalence was similar between groups, with 40/189 (21.2%) children in the IHAT group developing at least one episode of moderate-severe diarrhoea over the 85 days intervention, compared with 47/198 (23.7%) in the FeSO4 group (OR 1.18, 80% CI 0.86-1.62) and 40/195 (20.5%) in the placebo group (OR 0.96, 80% CI 0.7-1.33, PP population). Incidence density of moderate-severe diarrhoea was 2.66 in the IHAT group and 3.42 in the FeSO4 group (RR 0.76, 80% CI 0.59-0.99, CC-ITT population).There were 143/211 (67.8%) children with adverse events (AEs) in the IHAT group, 146/212 (68.9%) in the FeSO4 group and 143/214 (66.8%) in the placebo group. There were overall 213 diarrhoea-related AEs; 35 (28.5%) cases reported in the IHAT group compared with 51 (41.5%) cases in the FeSO4 group and 37 (30.1%) cases in the placebo group.
Interpretation: In this first Phase II study conducted in young children with IDA, IHAT showed sufficient non-inferiority compared to standard-of-care FeSO4, in terms of ID correction and haemoglobin response, to warrant a definitive Phase III trial. In addition, IHAT had lower incidence of moderate-severe diarrhoea than FeSO4, with no increased adverse events in comparison with placebo.
Funding: The Bill & Melinda Gates Foundation (OPP1140952).
Keywords: Anaemia; Children; Clinical trial; Iron deficiency; Iron hydroxide adipate tartrate (IHAT); Iron supplementation; Phase II.
© 2023 The Authors.
Conflict of interest statement
D.I.A.P., N.F. and J.J.P. are inventors of the IHAT iron supplementation technology, for which they could receive future awards to inventors through the MRC Awards to Inventor scheme. They are also scientific advisors to Nemysis Ltd, who now hold the license for IHAT. They have received honoraria or consultancy fees from some or all of: Vifor Pharma UK, Shield Therapeutics, Entia Ltd, Danone Nutricia, UN Food and Agriculture Organisation (FAO) and Nemysis Ltd, for work concerning iron in health. D.I.A.P. has since moved to full employment in the iron and health industry with Vifor Pharma UK but all work pertaining to this publication was conducted whilst at the University of Cambridge and MRC Unit The Gambia at LSHTM. Nemysis Ltd paid consulting fees to J.W.’s Institution. Notwithstanding, the authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- James S.L., Abate D., Abate K.H., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. - PMC - PubMed
-
- World Health Organization . WHO Report; Geneva: 2011. The Global Prevalence of Anaemia in 2011.
-
- Stoltzfus R., Mullany L., Black R. In: Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. Ezzati M., Lopez A., Rodgers A., Murray C., editors. WHO; Geneva: 2004. Iron deficiency anemia; pp. 163–209.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous