Public health impact of recombinant zoster vaccine for prevention of herpes zoster in US adults immunocompromised due to cancer
- PMID: 36880669
- PMCID: PMC10038038
- DOI: 10.1080/21645515.2023.2167907
Public health impact of recombinant zoster vaccine for prevention of herpes zoster in US adults immunocompromised due to cancer
Abstract
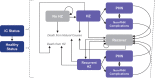
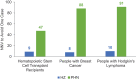
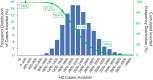
Individuals who are immunocompromised (IC) due to therapy or underlying disease are at increased risk of herpes zoster (HZ). This study evaluates the public health impact of recombinant zoster vaccine (RZV) relative to no HZ vaccination for the prevention of HZ among adults aged ≥18 years diagnosed with selected cancers in the United States (US). A static Markov model was used to simulate three cohorts of individuals who are IC with cancer (time horizon of 30 years; one-year cycle length): hematopoietic stem cell transplant (HSCT) recipients, patients with breast cancer (BC; a solid tumor example), and patients with Hodgkin's lymphoma (HL; a hematological malignancy example). Cohort sizes reflect the estimated annual incidence of each condition in the US population (19,671 HSCT recipients, 279,100 patients with BC, and 8,480 patients with HL). Vaccination with RZV resulted in 2,297; 38,068; and 848 fewer HZ cases for HSCT recipients, patients with BC, and patients with HL, respectively (each versus no vaccine). Vaccination with RZV also resulted in 422; 3,184; and 93 fewer postherpetic neuralgia cases for HSCT, BC, and HL, respectively. Analyses estimated the quality-adjusted life years gained to be 109, 506, and 17 for HSCT, BC, and HL, respectively. To prevent one HZ case, the number needed to vaccinate was 9, 8, and 10, for HSCT, BC, and HL, respectively. These results suggest RZV vaccination may be an effective option to significantly reduce HZ disease burden among patients diagnosed with selected cancers in the US.
Keywords: Herpes zoster; Hodgkin disease; breast neoplasms; hematopoietic stem cell transplantation; herpes zoster vaccine; neoplasms; public health.
Plain language summary
Shingles cases can be prevented by recombinant zoster vaccine (RZV). People who have a weakened immune system (immunocompromised) due to disease or therapy are more likely to develop shingles. For example, shingles occurs in nearly a quarter of patients receiving immunosuppressive treatment for blood cancers. To estimate the public health impact of vaccination against shingles in people who are immunocompromised due to cancer in the United States (US), we used a model to simulate groups with selected types of cancer. The results indicate vaccination with RZV can significantly reduce shingles cases and related complications among these groups in the US.
Conflict of interest statement
DC, AS, EML and SP are employees of the GSK group of companies. DC, AS, EML and SP report holding shares in the GSK group of companies. JC and KAH report payments to RTI Health Solutions from the GSK group of companies for the conduct of this study. BJP reports holding shares in the GSK group of companies and is a former employee of the GSK group of companies. SL and CFC declare to have received consulting fees from the GSK group of companies. All authors declare no other financial and non-financial relationships and activities.
Figures
Similar articles
-
Public health impact of herpes zoster vaccination on older adults in Hong Kong.Hum Vaccin Immunother. 2023 Dec 31;19(1):2176065. doi: 10.1080/21645515.2023.2176065. Epub 2023 Feb 28. Hum Vaccin Immunother. 2023. PMID: 36854447 Free PMC article.
-
Public health impact of herpes zoster vaccination on older adults in Singapore: a modeling study.Hum Vaccin Immunother. 2024 Dec 31;20(1):2348839. doi: 10.1080/21645515.2024.2348839. Epub 2024 May 28. Hum Vaccin Immunother. 2024. PMID: 38804600 Free PMC article.
-
Cost-effectiveness and public health impact of recombinant zoster vaccine versus no herpes zoster vaccination in selected populations of immunocompromised adults in Canada.BMC Health Serv Res. 2025 Apr 25;25(1):604. doi: 10.1186/s12913-025-12550-x. BMC Health Serv Res. 2025. PMID: 40281614 Free PMC article.
-
Recombinant zoster vaccine in immunocompetent and immunocompromised adults: A review of clinical studies.Hum Vaccin Immunother. 2023 Dec 15;19(3):2278362. doi: 10.1080/21645515.2023.2278362. Epub 2023 Nov 15. Hum Vaccin Immunother. 2023. PMID: 37965770 Free PMC article. Review.
-
Herpes zoster in Belgium: a new solution to an old problem.Acta Clin Belg. 2024 Jun;79(3):205-216. doi: 10.1080/17843286.2024.2350258. Epub 2024 May 23. Acta Clin Belg. 2024. PMID: 38781037 Review.
Cited by
-
Herpes Zoster Vaccination: Insights into Efficacy, Safety, and Guidelines.Vaccines (Basel). 2025 Apr 28;13(5):477. doi: 10.3390/vaccines13050477. Vaccines (Basel). 2025. PMID: 40432089 Free PMC article. Review.
-
The Potential Impact of Increased Recombinant Zoster Vaccine Uptake in Older Adults Worldwide.Infect Dis Ther. 2025 Jun;14(6):1327-1341. doi: 10.1007/s40121-025-01161-y. Epub 2025 May 21. Infect Dis Ther. 2025. PMID: 40399558 Free PMC article.
-
The Association Between Herpes Zoster, Antiherpetic Therapies, and Alzheimer's Disease: A Comprehensive Systematic Review.Pharmaceuticals (Basel). 2025 May 14;18(5):722. doi: 10.3390/ph18050722. Pharmaceuticals (Basel). 2025. PMID: 40430540 Free PMC article. Review.
-
Hospitalization burden related to herpes zoster infection during the COVID-19 pandemic in Spain (2020-2021).Hum Vaccin Immunother. 2023 Aug;19(2):2256047. doi: 10.1080/21645515.2023.2256047. Epub 2023 Oct 6. Hum Vaccin Immunother. 2023. PMID: 37799065 Free PMC article.
-
Practices, Attitudes, and Knowledge Regarding Recombinant Zoster Vaccine Among Family Medicine Residents in Riyadh, Saudi Arabia.Cureus. 2024 Aug 6;16(8):e66301. doi: 10.7759/cureus.66301. eCollection 2024 Aug. Cureus. 2024. PMID: 39238674 Free PMC article.
References
-
- CDC . Shingles (herpes zoster): clinical overview; 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical