Improved Bacterial Single-Cell RNA-Seq through Automated MATQ-Seq and Cas9-Based Removal of rRNA Reads
- PMID: 36880749
- PMCID: PMC10127585
- DOI: 10.1128/mbio.03557-22
Improved Bacterial Single-Cell RNA-Seq through Automated MATQ-Seq and Cas9-Based Removal of rRNA Reads
Abstract
Bulk RNA sequencing technologies have provided invaluable insights into host and bacterial gene expression and associated regulatory networks. Nevertheless, the majority of these approaches report average expression across cell populations, hiding the true underlying expression patterns that are often heterogeneous in nature. Due to technical advances, single-cell transcriptomics in bacteria has recently become reality, allowing exploration of these heterogeneous populations, which are often the result of environmental changes and stressors. In this work, we have improved our previously published bacterial single-cell RNA sequencing (scRNA-seq) protocol that is based on multiple annealing and deoxycytidine (dC) tailing-based quantitative scRNA-seq (MATQ-seq), achieving a higher throughput through the integration of automation. We also selected a more efficient reverse transcriptase, which led to reduced cell loss and higher workflow robustness. Moreover, we successfully implemented a Cas9-based rRNA depletion protocol into the MATQ-seq workflow. Applying our improved protocol on a large set of single Salmonella cells sampled over different growth conditions revealed improved gene coverage and a higher gene detection limit compared to our original protocol and allowed us to detect the expression of small regulatory RNAs, such as GcvB or CsrB at a single-cell level. In addition, we confirmed previously described phenotypic heterogeneity in Salmonella in regard to expression of pathogenicity-associated genes. Overall, the low percentage of cell loss and high gene detection limit makes the improved MATQ-seq protocol particularly well suited for studies with limited input material, such as analysis of small bacterial populations in host niches or intracellular bacteria. IMPORTANCE Gene expression heterogeneity among isogenic bacteria is linked to clinically relevant scenarios, like biofilm formation and antibiotic tolerance. The recent development of bacterial single-cell RNA sequencing (scRNA-seq) enables the study of cell-to-cell variability in bacterial populations and the mechanisms underlying these phenomena. Here, we report a scRNA-seq workflow based on MATQ-seq with increased robustness, reduced cell loss, and improved transcript capture rate and gene coverage. Use of a more efficient reverse transcriptase and the integration of an rRNA depletion step, which can be adapted to other bacterial single-cell workflows, was instrumental for these improvements. Applying the protocol to the foodborne pathogen Salmonella, we confirmed transcriptional heterogeneity across and within different growth phases and demonstrated that our workflow captures small regulatory RNAs at a single-cell level. Due to low cell loss and high transcript capture rates, this protocol is uniquely suited for experimental settings in which the starting material is limited, such as infected tissues.
Keywords: Cas9; DASH; MATQ-seq; Salmonella enterica; gene expression heterogeneity; rRNA depletion; single-cell RNA-seq.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
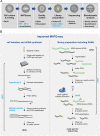


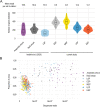
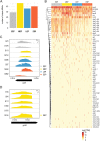

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

