Deep-Learning for Epicardial Adipose Tissue Assessment With Computed Tomography: Implications for Cardiovascular Risk Prediction
- PMID: 36881425
- PMCID: PMC10663979
- DOI: 10.1016/j.jcmg.2022.11.018
Deep-Learning for Epicardial Adipose Tissue Assessment With Computed Tomography: Implications for Cardiovascular Risk Prediction
Abstract
Background: Epicardial adipose tissue (EAT) volume is a marker of visceral obesity that can be measured in coronary computed tomography angiograms (CCTA). The clinical value of integrating this measurement in routine CCTA interpretation has not been documented.
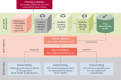
Objectives: This study sought to develop a deep-learning network for automated quantification of EAT volume from CCTA, test it in patients who are technically challenging, and validate its prognostic value in routine clinical care.
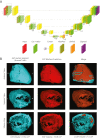
Methods: The deep-learning network was trained and validated to autosegment EAT volume in 3,720 CCTA scans from the ORFAN (Oxford Risk Factors and Noninvasive Imaging Study) cohort. The model was tested in patients with challenging anatomy and scan artifacts and applied to a longitudinal cohort of 253 patients post-cardiac surgery and 1,558 patients from the SCOT-HEART (Scottish Computed Tomography of the Heart) Trial, to investigate its prognostic value.
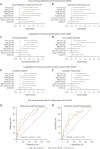
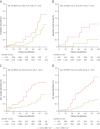
Results: External validation of the deep-learning network yielded a concordance correlation coefficient of 0.970 for machine vs human. EAT volume was associated with coronary artery disease (odds ratio [OR] per SD increase in EAT volume: 1.13 [95% CI: 1.04-1.30]; P = 0.01), and atrial fibrillation (OR: 1.25 [95% CI: 1.08-1.40]; P = 0.03), after correction for risk factors (including body mass index). EAT volume predicted all-cause mortality (HR per SD: 1.28 [95% CI: 1.10-1.37]; P = 0.02), myocardial infarction (HR: 1.26 [95% CI:1.09-1.38]; P = 0.001), and stroke (HR: 1.20 [95% CI: 1.09-1.38]; P = 0.02) independently of risk factors in SCOT-HEART (5-year follow-up). It also predicted in-hospital (HR: 2.67 [95% CI: 1.26-3.73]; P ≤ 0.01) and long-term post-cardiac surgery atrial fibrillation (7-year follow-up; HR: 2.14 [95% CI: 1.19-2.97]; P ≤ 0.01).
Conclusions: Automated assessment of EAT volume is possible in CCTA, including in patients who are technically challenging; it forms a powerful marker of metabolically unhealthy visceral obesity, which could be used for cardiovascular risk stratification.
Keywords: adipose tissue; atherosclerosis; atrial fibrillation; computed tomography; deep-learning; visceral fat.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This study received support from the British Heart Foundation (grant TG/19/2/34831) and the European Union Commission Horizon 2020 program via the Machine Learning Artificial Intelligence Early Detection Stroke Atrial Fibrillation (MAESTRIA) Consortium (grant 965286). Drs Siddique, Tomlins, and Shirodaria are employees of Caristo Diagnostics Ltd. Dr Williams has received support from the British Heart Foundation (grant FS/ICRF/20/26002); and has served on the Speakers Bureau for Canon Medical Systems. Dr Adlam has received support from the Leicester National Institute of Health Research Biomedical Research Centre; has received research funding and in-kind support for unrelated research from AstraZeneca Inc; has received an educational grant from Abbott Vascular Inc to support a clinical research fellow for unrelated research; and has also conducted consultancy for GE Inc to support research funds for unrelated research. Drs Shirodaria, Neubauer, Channon, and Antoniades are founders, shareholders, and directors of Caristo Diagnostics Ltd, a CT-image analysis company. Dr Antoniades has received support from the British Heart Foundation (grants CH/F/21/90009, TG/19/2/34831, and RG/F/21/110040), Innovate UK (grant 104472), and the National Consortium of Intelligent Medical Imaging through the Industry Strategy Challenge Fund (Innovate UK grant 104688); and is also the inventor of patents US10,695,023B2, PCT/GB2017/053262, GB2018/1818049.7, GR20180100490, and GR20180100510, which are licensed through exclusive license to Caristo Diagnostics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures






Comment in
-
Artificial Intelligence for Assessment of Epicardial Adipose Tissue on Coronary CT Angiography.JACC Cardiovasc Imaging. 2023 Jun;16(6):817-819. doi: 10.1016/j.jcmg.2022.12.004. Epub 2023 Feb 8. JACC Cardiovasc Imaging. 2023. PMID: 36881422 No abstract available.
References
-
- Knuuti J., Wijns W., Saraste A., et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. - PubMed
-
- Gulati M., Levy P.D., Mukherjee D., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78(22):e187–e285. - PubMed
-
- Oikonomou E.K., West H.W., Antoniades C. Cardiac computed tomography: assessment of coronary inflammation and other plaque features. Arterioscler Thromb Vasc Biol. 2019;39(11):2207–2219. - PubMed
-
- Oikonomou E.K., Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol. 2019;16(2):83–99. - PubMed
Publication types
MeSH terms
Grants and funding
- SP/17/16/33519/BHF_/British Heart Foundation/United Kingdom
- CH/F/21/90009/BHF_/British Heart Foundation/United Kingdom
- RG/F/21/110040/BHF_/British Heart Foundation/United Kingdom
- FS/16/15/32047/BHF_/British Heart Foundation/United Kingdom
- FS/ICRF/20/26002/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

