Vertical growth dynamics of biofilms
- PMID: 36881625
- PMCID: PMC10089195
- DOI: 10.1073/pnas.2214211120
Vertical growth dynamics of biofilms
Abstract
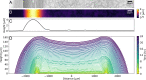
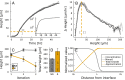
During the biofilm life cycle, bacteria attach to a surface and then reproduce, forming crowded, growing communities. Many theoretical models of biofilm growth dynamics have been proposed; however, difficulties in accurately measuring biofilm height across relevant time and length scales have prevented testing these models, or their biophysical underpinnings, empirically. Using white light interferometry, we measure the heights of microbial colonies with nanometer precision from inoculation to their final equilibrium height, producing a detailed empirical characterization of vertical growth dynamics. We propose a heuristic model for vertical growth dynamics based on basic biophysical processes inside a biofilm: diffusion and consumption of nutrients and growth and decay of the colony. This model captures the vertical growth dynamics from short to long time scales (10 min to 14 d) of diverse microorganisms, including bacteria and fungi.
Keywords: biofilms; biophysics; super-resolution.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Flemming H. C., et al. , Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575 (2016). - PubMed
-
- Hall-Stoodley L., Costerton J. W., Stoodley P., Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2, 95–108 (2004). - PubMed
-
- G. Lambert, A. Bergman, Q. Zhang, D. Bortz, R. Austin, Physics of biofilms: The initial stages of biofilm formation and dynamics. New J. Phys. 16 (2014).
-
- Nadell C. D., Drescher K., Foster K. R., Spatial structure, cooperation and competition in biofilms. Nat. Rev. Microbiol. 14, 589–600 (2016). - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

