Early development of respiratory motor circuits in larval zebrafish (Danio rerio)
- PMID: 36881713
- PMCID: PMC10081962
- DOI: 10.1002/cne.25467
Early development of respiratory motor circuits in larval zebrafish (Danio rerio)
Abstract
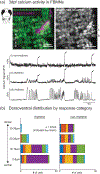
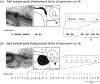
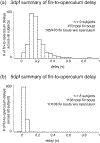
Rhythm-generating circuits in the vertebrate hindbrain form synaptic connections with cranial and spinal motor neurons, to generate coordinated, patterned respiratory behaviors. Zebrafish provide a uniquely tractable model system to investigate the earliest stages in respiratory motor circuit development in vivo. In larval zebrafish, respiratory behaviors are carried out by muscles innervated by cranial motor neurons-including the facial branchiomotor neurons (FBMNs), which innervate muscles that move the jaw, buccal cavity, and operculum. However, it is unclear when FBMNs first receive functional synaptic input from respiratory pattern-generating neurons, and how the functional output of the respiratory motor circuit changes across larval development. In the current study, we used behavior and calcium imaging to determine how early FBMNs receive functional synaptic inputs from respiratory pattern-generating networks in larval zebrafish. Zebrafish exhibited patterned operculum movements by 3 days postfertilization (dpf), though this behavior became more consistent at 4 and 5 dpf. Also by 3dpf, FBMNs fell into two distinct categories ("rhythmic" and "nonrhythmic"), based on patterns of neural activity. These two neuron categories were arranged differently along the dorsoventral axis, demonstrating that FBMNs have already established dorsoventral topography by 3 dpf. Finally, operculum movements were coordinated with pectoral fin movements at 3 dpf, indicating that the operculum behavioral pattern was driven by synaptic input. Taken together, this evidence suggests that FBMNs begin to receive initial synaptic input from a functional respiratory central pattern generator at or prior to 3 dpf. Future studies will use this model to study mechanisms of normal and abnormal respiratory circuit development.
Keywords: facial branchiomotor neurons; motor circuits; respiratory behavior; respiratory circuits; respiratory development; zebrafish.
© 2023 The Authors. The Journal of Comparative Neurology published by Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURE
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

