Dynamic interplay between RPL3- and RPL3L-containing ribosomes modulates mitochondrial activity in the mammalian heart
- PMID: 36882085
- PMCID: PMC10287911
- DOI: 10.1093/nar/gkad121
Dynamic interplay between RPL3- and RPL3L-containing ribosomes modulates mitochondrial activity in the mammalian heart
Abstract
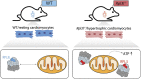
The existence of naturally occurring ribosome heterogeneity is now a well-acknowledged phenomenon. However, whether this heterogeneity leads to functionally diverse 'specialized ribosomes' is still a controversial topic. Here, we explore the biological function of RPL3L (uL3L), a ribosomal protein (RP) paralogue of RPL3 (uL3) that is exclusively expressed in skeletal muscle and heart tissues, by generating a viable homozygous Rpl3l knockout mouse strain. We identify a rescue mechanism in which, upon RPL3L depletion, RPL3 becomes up-regulated, yielding RPL3-containing ribosomes instead of RPL3L-containing ribosomes that are typically found in cardiomyocytes. Using both ribosome profiling (Ribo-seq) and a novel orthogonal approach consisting of ribosome pulldown coupled to nanopore sequencing (Nano-TRAP), we find that RPL3L modulates neither translational efficiency nor ribosome affinity towards a specific subset of transcripts. In contrast, we show that depletion of RPL3L leads to increased ribosome-mitochondria interactions in cardiomyocytes, which is accompanied by a significant increase in ATP levels, potentially as a result of fine-tuning of mitochondrial activity. Our results demonstrate that the existence of tissue-specific RP paralogues does not necessarily lead to enhanced translation of specific transcripts or modulation of translational output. Instead, we reveal a complex cellular scenario in which RPL3L modulates the expression of RPL3, which in turn affects ribosomal subcellular localization and, ultimately, mitochondrial activity.
Plain language summary
Ribosomes are macromolecular machines responsible for protein synthesis in all living beings. Recent studies have shown that ribosomes can be heterogeneous in their structure, possibly leading to a specialized function. Here, we focus on RPL3L, a ribosomal protein expressed exclusively in striated muscles. We find that the deletion of the Rpl3l gene in a mouse model triggers a compensation mechanism, in which the missing RPL3L protein is replaced by its paralogue, RPL3. Furthermore, we find that RPL3-containing ribosomes establish closer interactions with mitochondria, cellular organelles responsible for energy production, leading to higher energy production when compared with RPL3L-containing ribosomes. Finally, we show that the RPL3–RPL3L compensation mechanism is also triggered in heart disease conditions, such as hypertrophy and myocardial infarction.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






References
-
- Williams M.E., Sussex I.M.. Developmental regulation of ribosomal protein L16 genes in Arabidopsis thaliana. Plant J. 1995; 8:65–76. - PubMed

