Efficacy and safety of GLP-1 receptor agonists versus SGLT-2 inhibitors in overweight/obese patients with or without diabetes mellitus: a systematic review and network meta-analysis
- PMID: 36882248
- PMCID: PMC10008474
- DOI: 10.1136/bmjopen-2022-061807
Efficacy and safety of GLP-1 receptor agonists versus SGLT-2 inhibitors in overweight/obese patients with or without diabetes mellitus: a systematic review and network meta-analysis
Abstract
Objective: To compare the efficacy and safety between and within glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter 2 inhibitors (SGLT-2is) in overweight or obese adults with or without diabetes mellitus.
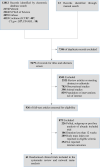
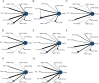
Methods: PubMed, ISI Web of Science, Embase and Cochrane Central Register of Controlled Trials database were comprehensively searched to identify randomised controlled trials (RCTs) of effects of GLP-1RAs and SGLT-2is in overweight or obese participants from inception to 16 January 2022. The efficacy outcomes were the changes of body weight, glucose level and blood pressure. The safety outcomes were serious adverse events and discontinuation due to adverse events. The mean differences, ORs, 95% credible intervals (95% CI), the surface under the cumulative ranking were evaluated for each outcome by network meta-analysis.
Results: Sixty-one RCTs were included in our analysis. Both GLP-1RAs and SGLT-2is conferred greater extents in body weight reduction, achieving at least 5% wt loss, HbA1c and fasting plasma glucose decrease compared with placebo. GLP-1RAs was superior to SGLT-2is in HbA1c reduction (MD: -0.39%, 95% CI -0.70 to -0.08). GLP-1RAs had high risk of adverse events, while SGLT-2is were relatively safe. Based on intraclass comparison, semaglutide 2.4 mg was among the most effective interventions in losing body weight (MD: -11.51 kg, 95% CI -12.83 to -10.21), decreasing HbA1c (MD: -1.49%, 95% CI -2.07 to -0.92) and fasting plasma glucose (MD: -2.15 mmol/L, 95% CI -2.83 to -1.59), reducing systolic blood pressure (MD: -4.89 mm Hg, 95% CI -6.04 to -3.71) and diastolic blood pressure (MD: -1.59 mm Hg, 95% CI -2.37 to -0.86) with moderate certainty evidences, while it was associated with high risk of adverse events.
Conclusions: Semaglutide 2.4 mg showed the greatest effects on losing body weight, controlling glycaemic level and reducing blood pressure while it was associated with high risk of adverse events.PROSPERO registration numberCRD42021258103.
Keywords: DIABETES & ENDOCRINOLOGY; Diabetes & endocrinology; General diabetes.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
-
- Health Organization (WHO) . Obesity, retrieved on 10 september 2017. n.d. Available: http://www.euro.who.int/en/health-topics/noncommunicable-diseases/obesity
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
