Surfactants or scaffolds? RNAs of varying lengths control the thermodynamic stability of condensates differently
- PMID: 36883003
- PMCID: PMC10398262
- DOI: 10.1016/j.bpj.2023.03.006
Surfactants or scaffolds? RNAs of varying lengths control the thermodynamic stability of condensates differently
Abstract
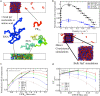
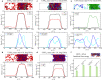
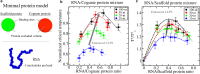
Biomolecular condensates, thought to form via liquid-liquid phase separation of intracellular mixtures, are multicomponent systems that can include diverse types of proteins and RNAs. RNA is a critical modulator of RNA-protein condensate stability, as it induces an RNA concentration-dependent reentrant phase transition-increasing stability at low RNA concentrations and decreasing it at high concentrations. Beyond concentration, RNAs inside condensates can be heterogeneous in length, sequence, and structure. Here, we use multiscale simulations to understand how different RNA parameters interact with one another to modulate the properties of RNA-protein condensates. To do so, we perform residue/nucleotide resolution coarse-grained molecular dynamics simulations of multicomponent RNA-protein condensates containing RNAs of different lengths and concentrations, and either FUS or PR25 proteins. Our simulations reveal that RNA length regulates the reentrant phase behavior of RNA-protein condensates: increasing RNA length sensitively rises the maximum value that the critical temperature of the mixture reaches, and the maximum concentration of RNA that the condensate can incorporate before beginning to become unstable. Strikingly, RNAs of different lengths are organized heterogeneously inside condensates, which allows them to enhance condensate stability via two distinct mechanisms: shorter RNA chains accumulate at the condensate's surface acting as natural biomolecular surfactants, while longer RNA chains concentrate inside the core to saturate their bonds and enhance the density of molecular connections in the condensate. Using a patchy particle model, we additionally demonstrate that the combined impact of RNA length and concentration on condensate properties is dictated by the valency, binding affinity, and polymer length of the various biomolecules involved. Our results postulate that diversity on RNA parameters within condensates allows RNAs to increase condensate stability by fulfilling two different criteria: maximizing enthalpic gain and minimizing interfacial free energy; hence, RNA diversity should be considered when assessing the impact of RNA on biomolecular condensates regulation.
Copyright © 2023 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Hyman A.A., Weber C.A., Jülicher F. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 2014;30:39–58. - PubMed
-
- Alberti S. Phase separation in biology. Curr. Biol. 2017;27:R1097–R1102. - PubMed
-
- Brangwynne C.P., Eckmann C.R., et al. Hyman A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

