Manipulation of iron status on cerebral blood flow at high altitude in lowlanders and adapted highlanders
- PMID: 36883428
- PMCID: PMC10291452
- DOI: 10.1177/0271678X231152734
Manipulation of iron status on cerebral blood flow at high altitude in lowlanders and adapted highlanders
Abstract
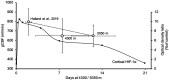
Cerebral blood flow (CBF) increases during hypoxia to counteract the reduction in arterial oxygen content. The onset of tissue hypoxemia coincides with the stabilization of hypoxia-inducible factor (HIF) and transcription of downstream HIF-mediated processes. It has yet to be determined, whether HIF down- or upregulation can modulate hypoxic vasodilation of the cerebral vasculature. Therefore, we examined whether: 1) CBF would increase with iron depletion (via chelation) and decrease with repletion (via iron infusion) at high-altitude, and 2) explore whether genotypic advantages of highlanders extend to HIF-mediated regulation of CBF. In a double-blinded and block-randomized design, CBF was assessed in 82 healthy participants (38 lowlanders, 20 Sherpas and 24 Andeans), before and after the infusion of either: iron(III)-hydroxide sucrose, desferrioxamine or saline. Across both lowlanders and highlanders, baseline iron levels contributed to the variability in cerebral hypoxic reactivity at high altitude (R2 = 0.174, P < 0.001). At 5,050 m, CBF in lowlanders and Sherpa were unaltered by desferrioxamine or iron. At 4,300 m, iron infusion led to 4 ± 10% reduction in CBF (main effect of time p = 0.043) in lowlanders and Andeans. Iron status may provide a novel, albeit subtle, influence on CBF that is potentially dependent on the severity and length-of-stay at high altitude.
Keywords: Iron; cerebral blood flow; high altitude; high-altitude residents; hypoxia.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Severinghaus JW, Chiodi H, Eger EI, et al.. Cerebral blood flow in man at high altitude. Role of cerebrospinal fluid pH in normalization of flow in chronic hypocapnia. Circ Res 1966; 19: 274–282. - PubMed
-
- Hoiland RL, Howe CA, Carter HH, et al.. UBC-Nepal expedition: phenotypical evidence for evolutionary adaptation in the control of cerebral blood flow and oxygen delivery at high altitude. J Physiol 2019; 597: 2993–3008. - PubMed
-
- Huang SY, Moore LG, McCullough RE, et al.. Internal carotid and vertebral arterial flow velocity in men at high altitude. J Appl Physiol (1985) 1987; 63: 395–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

