Nitrative Modification of Caveolin-3: A Novel Mechanism of Cardiac Insulin Resistance and a Potential Therapeutic Target Against Ischemic Heart Failure in Prediabetic Animals
- PMID: 36883479
- PMCID: PMC10085855
- DOI: 10.1161/CIRCULATIONAHA.122.063073
Nitrative Modification of Caveolin-3: A Novel Mechanism of Cardiac Insulin Resistance and a Potential Therapeutic Target Against Ischemic Heart Failure in Prediabetic Animals
Erratum in
-
Correction to: Nitrative Modification of Caveolin-3: A Novel Mechanism of Cardiac Insulin Resistance and a Potential Therapeutic Target against Ischemic Heart Failure in Prediabetic Animals.Circulation. 2024 Jul 9;150(2):e63. doi: 10.1161/CIR.0000000000001270. Epub 2024 Jul 8. Circulation. 2024. PMID: 38976615 No abstract available.
Abstract
Background: Myocardial insulin resistance is a hallmark of diabetic cardiac injury. However, the underlying molecular mechanisms remain unclear. Recent studies demonstrate that the diabetic heart is resistant to other cardioprotective interventions, including adiponectin and preconditioning. The "universal" resistance to multiple therapeutic interventions suggests impairment of the requisite molecule(s) involved in broad prosurvival signaling cascades. Cav (Caveolin) is a scaffolding protein coordinating transmembrane signaling transduction. However, the role of Cav3 in diabetic impairment of cardiac protective signaling and diabetic ischemic heart failure is unknown.
Methods: Wild-type and gene-manipulated mice were fed a normal diet or high-fat diet for 2 to 12 weeks and subjected to myocardial ischemia and reperfusion. Insulin cardioprotection was determined.
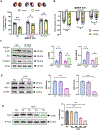
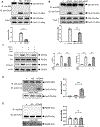
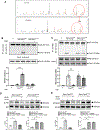
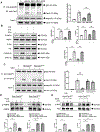
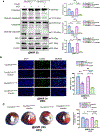
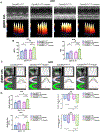
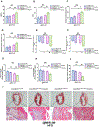
Results: Compared with the normal diet group, the cardioprotective effect of insulin was significantly blunted as early as 4 weeks of high-fat diet feeding (prediabetes), a time point where expression levels of insulin-signaling molecules remained unchanged. However, Cav3/insulin receptor-β complex formation was significantly reduced. Among multiple posttranslational modifications altering protein/protein interaction, Cav3 (not insulin receptor-β) tyrosine nitration is prominent in the prediabetic heart. Treatment of cardiomyocytes with 5-amino-3-(4-morpholinyl)-1,2,3-oxadiazolium chloride reduced the signalsome complex and blocked insulin transmembrane signaling. Mass spectrometry identified Tyr73 as the Cav3 nitration site. Phenylalanine substitution of Tyr73 (Cav3Y73F) abolished 5-amino-3-(4-morpholinyl)-1,2,3-oxadiazolium chloride-induced Cav3 nitration, restored Cav3/insulin receptor-β complex, and rescued insulin transmembrane signaling. It is most important that adeno-associated virus 9-mediated cardiomyocyte-specific Cav3Y73F reexpression blocked high-fat diet-induced Cav3 nitration, preserved Cav3 signalsome integrity, restored transmembrane signaling, and rescued insulin-protective action against ischemic heart failure. Last, diabetic nitrative modification of Cav3 at Tyr73 also reduced Cav3/AdipoR1 complex formation and blocked adiponectin cardioprotective signaling.
Conclusions: Nitration of Cav3 at Tyr73 and resultant signal complex dissociation results in cardiac insulin/adiponectin resistance in the prediabetic heart, contributing to ischemic heart failure progression. Early interventions preserving Cav3-centered signalsome integrity is an effective novel strategy against diabetic exacerbation of ischemic heart failure.
Keywords: caveolin 3; diabetes mellitus; protein processing, posttranslational; reperfusion injury.
Conflict of interest statement
Disclosures
The authors declare no competing interests, financial or otherwise.
Figures

Comment in
-
Caveolin-3 Nitration Drives Insulin Resistance in Prediabetic Hearts.Circulation. 2023 Apr 11;147(15):1180-1182. doi: 10.1161/CIRCULATIONAHA.123.064250. Epub 2023 Apr 10. Circulation. 2023. PMID: 37036910 Free PMC article. No abstract available.
References
-
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. - PubMed
-
- Randhawa VK, Dhanvantari S and Connelly KA. How Diabetes and Heart Failure Modulate Each Other and Condition Management. Can J Cardiol. 2021;37:595–608. - PubMed
-
- Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, McCarren M, Duckworth WC and Emanuele NV. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–2206. - PubMed
-
- Abel ED. Myocardial insulin resistance and cardiac complications of diabetes. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:219–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

