Advance in placenta drug delivery: concern for placenta-originated disease therapy
- PMID: 36883905
- PMCID: PMC10003143
- DOI: 10.1080/10717544.2023.2184315
Advance in placenta drug delivery: concern for placenta-originated disease therapy
Abstract
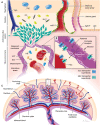
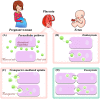
In the therapy of placenta-originated diseases during pregnancy, the main challenges are fetal exposure to drugs, which can pass through the placenta and cause safety concerns for fetal development. The design of placenta-resident drug delivery system is an advantageous method to minimize fetal exposure as well as reduce adverse maternal off-target effects. By utilizing the placenta as a biological barrier, the placenta-resident nanodrugs could be trapped in the local placenta to concentrate on the treatment of this abnormal originated tissue. Therefore, the success of such systems largely depends on the placental retention capacity. This paper expounds on the transport mechanism of nanodrugs in the placenta, analyzes the factors that affect the placental retention of nanodrugs, and summarizes the advantages and concerns of current nanoplatforms in the treatment of placenta-originated diseases. In general, this review aims to provide a theoretical basis for the construction of placenta-resident drug delivery systems, which will potentially enable safe and efficient clinical treatment for placenta-originated diseases in the future.
Keywords: Placenta; drug delivery; nanoplatforms; pregnancy; retention effect.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

Similar articles
-
Placental control of drug delivery.Adv Drug Deliv Rev. 2017 Jul 1;116:63-72. doi: 10.1016/j.addr.2016.08.002. Epub 2016 Aug 12. Adv Drug Deliv Rev. 2017. PMID: 27527665 Free PMC article. Review.
-
Placental drug transporters.Curr Drug Metab. 2004 Feb;5(1):125-31. doi: 10.2174/1389200043489171. Curr Drug Metab. 2004. PMID: 14965255 Review.
-
Drug transfer and metabolism by the human placenta.Clin Pharmacokinet. 2004;43(8):487-514. doi: 10.2165/00003088-200443080-00001. Clin Pharmacokinet. 2004. PMID: 15170365 Review.
-
Nanoparticulate drug delivery in pregnancy: placental passage and fetal exposure.Curr Pharm Biotechnol. 2011 May;12(5):731-42. doi: 10.2174/138920111795471010. Curr Pharm Biotechnol. 2011. PMID: 21342124 Review.
-
Pharmacotherapy in pregnancy; effect of ABC and SLC transporters on drug transport across the placenta and fetal drug exposure.J Drug Target. 2012 Nov;20(9):736-63. doi: 10.3109/1061186X.2012.716847. Epub 2012 Sep 20. J Drug Target. 2012. PMID: 22994411 Review.
Cited by
-
A pilot study on LC-MS/MS quantification of remifentanil, etomidate, and rocuronium in maternal and fetal serum microsamples.Sci Rep. 2025 Jul 16;15(1):25876. doi: 10.1038/s41598-025-09454-5. Sci Rep. 2025. PMID: 40670482 Free PMC article.
-
Actively Targeted Nanomedicines: A New Perspective for the Treatment of Pregnancy-Related Diseases.Reprod Sci. 2024 Sep;31(9):2560-2575. doi: 10.1007/s43032-024-01520-z. Epub 2024 Mar 29. Reprod Sci. 2024. PMID: 38553575 Review.
-
Amniotic fluid-derived stem cells: potential factories of natural and mimetic strategies for congenital malformations.Stem Cell Res Ther. 2024 Dec 5;15(1):466. doi: 10.1186/s13287-024-04082-8. Stem Cell Res Ther. 2024. PMID: 39639397 Free PMC article.
-
Fetus Exposure to Drugs and Chemicals: A Holistic Overview on the Assessment of Their Transport and Metabolism across the Human Placental Barrier.Diseases. 2024 Jun 1;12(6):114. doi: 10.3390/diseases12060114. Diseases. 2024. PMID: 38920546 Free PMC article. Review.
References
-
- Abdelghani M, Shao J, Le DH, et al. (2021). Self‐assembly or coassembly of multiresponsive histidine‐containing elastin‐like polypeptide block copolymers. Macromol Biosci 21:2100081. - PubMed
-
- Aengenheister L, Dugershaw BB, Manser P, et al. (2019). Investigating the accumulation and translocation of titanium dioxide nanoparticles with different surface modifications in static and dynamic human placental transfer models. Eur J Pharm Biopharm 142:488–18. - PubMed
-
- Aengenheister L, Favaro RR, Morales-Prieto DM, et al. (2021). Research on nanoparticles in human perfused placenta: State of the art and perspectives. Placenta 104:199–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
