Retrospective, Observational Studies for Estimating Vaccine Effects on the Secondary Attack Rate of SARS-CoV-2
- PMID: 36883907
- PMCID: PMC10505422
- DOI: 10.1093/aje/kwad046
Retrospective, Observational Studies for Estimating Vaccine Effects on the Secondary Attack Rate of SARS-CoV-2
Abstract
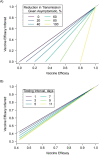
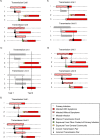
Coronavirus disease 2019 (COVID-19) vaccines are highly efficacious at preventing symptomatic infection, severe disease, and death. Most of the evidence that COVID-19 vaccines also reduce transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is based on retrospective, observational studies. Specifically, an increasing number of studies are evaluating vaccine effectiveness against the secondary attack rate of SARS-CoV-2 using data available in existing health-care databases or contact-tracing databases. Since these types of databases were designed for clinical diagnosis or management of COVID-19, they are limited in their ability to provide accurate information on infection, infection timing, and transmission events. We highlight challenges with using existing databases to identify transmission units and confirm potential SARS-CoV-2 transmission events. We discuss the impact of common diagnostic testing strategies, including event-prompted and infrequent testing, and illustrate their potential biases in estimating vaccine effectiveness against the secondary attack rate of SARS-CoV-2. We articulate the need for prospective observational studies of vaccine effectiveness against the SARS-CoV-2 secondary attack rate, and we provide design and reporting considerations for studies using retrospective databases.
Keywords: COVID-19; SARS-CoV-2; retrospective studies.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

