Presence of immunogenic alternatively spliced insulin gene product in human pancreatic delta cells
- PMID: 36884057
- PMCID: PMC10036285
- DOI: 10.1007/s00125-023-05882-y
Presence of immunogenic alternatively spliced insulin gene product in human pancreatic delta cells
Abstract
Aims/hypothesis: Transcriptome analyses revealed insulin-gene-derived transcripts in non-beta endocrine islet cells. We studied alternative splicing of human INS mRNA in pancreatic islets.
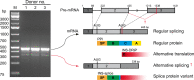
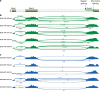
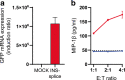
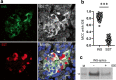
Methods: Alternative splicing of insulin pre-mRNA was determined by PCR analysis performed on human islet RNA and single-cell RNA-seq analysis. Antisera were generated to detect insulin variants in human pancreatic tissue using immunohistochemistry, electron microscopy and single-cell western blot to confirm the expression of insulin variants. Cytotoxic T lymphocyte (CTL) activation was determined by MIP-1β release.
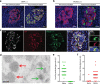
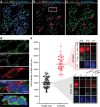
Results: We identified an alternatively spliced INS product. This variant encodes the complete insulin signal peptide and B chain and an alternative C-terminus that largely overlaps with a previously identified defective ribosomal product of INS. Immunohistochemical analysis revealed that the translation product of this INS-derived splice transcript was detectable in somatostatin-producing delta cells but not in beta cells; this was confirmed by light and electron microscopy. Expression of this alternatively spliced INS product activated preproinsulin-specific CTLs in vitro. The exclusive presence of this alternatively spliced INS product in delta cells may be explained by its clearance from beta cells by insulin-degrading enzyme capturing its insulin B chain fragment and a lack of insulin-degrading enzyme expression in delta cells.
Conclusions/interpretation: Our data demonstrate that delta cells can express an INS product derived from alternative splicing, containing both the diabetogenic insulin signal peptide and B chain, in their secretory granules. We propose that this alternative INS product may play a role in islet autoimmunity and pathology, as well as endocrine or paracrine function or islet development and endocrine destiny, and transdifferentiation between endocrine cells. INS promoter activity is not confined to beta cells and should be used with care when assigning beta cell identity and selectivity.
Data availability: The full EM dataset is available via www.nanotomy.org (for review: http://www.nanotomy.org/OA/Tienhoven2021SUB/6126-368/ ). Single-cell RNA-seq data was made available by Segerstolpe et al [13] and can be found at https://sandberglab.se/pancreas . The RNA and protein sequence of INS-splice was uploaded to GenBank (BankIt2546444 INS-splice OM489474).
Keywords: Alternative splicing; Alternative translation; Beta cell; Defective ribosomal product; Delta cell; Human islets of Langerhans; Insulin gene; Insulin-degrading enzyme; Type 1 diabetes.
© 2023. The Author(s).
Figures
Similar articles
-
Characterization of Human Pancreatic Islet Cells Using a Single-Cell Western Blot Platform.Pancreas. 2024 Nov-Dec 01;53(10):e818-e829. doi: 10.1097/MPA.0000000000002385. Epub 2024 Sep 12. Pancreas. 2024. PMID: 39259841
-
DTA-mediated targeted ablation revealed differential interdependence of endocrine cell lineages in early development of zebrafish pancreas.Differentiation. 2009 Nov;78(4):241-52. doi: 10.1016/j.diff.2009.05.009. Epub 2009 Jun 23. Differentiation. 2009. PMID: 19553000
-
Free fatty acid receptor 4 inhibitory signaling in delta cells regulates islet hormone secretion in mice.Mol Metab. 2021 Mar;45:101166. doi: 10.1016/j.molmet.2021.101166. Epub 2021 Jan 20. Mol Metab. 2021. PMID: 33484949 Free PMC article.
-
Paracrine signaling in islet function and survival.J Mol Med (Berl). 2020 Apr;98(4):451-467. doi: 10.1007/s00109-020-01887-x. Epub 2020 Feb 17. J Mol Med (Berl). 2020. PMID: 32067063 Free PMC article. Review.
-
Understanding Insulin in the Age of Precision Medicine and Big Data: Under-Explored Nature of Genomics.Biomolecules. 2023 Jan 30;13(2):257. doi: 10.3390/biom13020257. Biomolecules. 2023. PMID: 36830626 Free PMC article. Review.
Cited by
-
The Role of Somatostatin in the Gastrointestinal Tract.Biology (Basel). 2025 May 16;14(5):558. doi: 10.3390/biology14050558. Biology (Basel). 2025. PMID: 40427747 Free PMC article. Review.
-
Expanding the repertoire reveals recurrent, cryptic, and hematopoietic HLA class I minor histocompatibility antigens.Blood. 2024 May 2;143(18):1856-1872. doi: 10.1182/blood.2023022343. Blood. 2024. PMID: 38427583 Free PMC article.
-
Autoimmune CD8+ T cells in type 1 diabetes: from single-cell RNA sequencing to T-cell receptor redirection.Front Endocrinol (Lausanne). 2024 May 10;15:1377322. doi: 10.3389/fendo.2024.1377322. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38800484 Free PMC article. Review.
-
Induction of islet autoimmunity to defective ribosomal product of the insulin gene as neoantigen after anti-cancer immunotherapy leading to autoimmune diabetes.Front Immunol. 2024 Mar 26;15:1384406. doi: 10.3389/fimmu.2024.1384406. eCollection 2024. Front Immunol. 2024. PMID: 38596681 Free PMC article.
-
The Importance of Intra-Islet Communication in the Function and Plasticity of the Islets of Langerhans during Health and Diabetes.Int J Mol Sci. 2024 Apr 6;25(7):4070. doi: 10.3390/ijms25074070. Int J Mol Sci. 2024. PMID: 38612880 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

