Neuroprotective effect of tangeretin against chromium-induced acute brain injury in rats: targeting Nrf2 signaling pathway, inflammatory mediators, and apoptosis
- PMID: 36884189
- PMCID: PMC10229463
- DOI: 10.1007/s10787-023-01167-3
Neuroprotective effect of tangeretin against chromium-induced acute brain injury in rats: targeting Nrf2 signaling pathway, inflammatory mediators, and apoptosis
Abstract
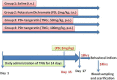
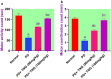
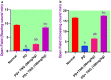
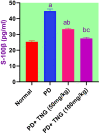
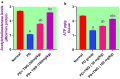
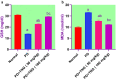
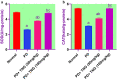
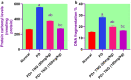
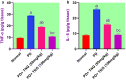
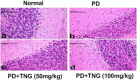
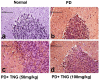
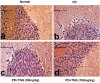
Potassium dichromate (PD) is an environmental xenobiotic commonly recognized as teratogenic, carcinogenic, and mutagenic in animals and humans. The present study was conducted to investigate the role of tangeretin (TNG) as a neuro-protective drug against PD-induced brain injury in rats. Thirty-two male adult Wistar rats were blindly divided into four groups (8 rats/group). The first group received saline intranasally (i.n.). The second group received a single dose of PD (2 mg/kg, i.n.). The third group received TNG (50 mg/kg; orally), for 14 days followed by i.n. of PD on the last day of the experiment. The fourth group received TNG (100 mg/kg; orally) for 14 days followed by i.n. of PD on the last day of the experiment. Behavioral indices were evaluated 18 h after PD administration. Neuro-biochemical indices and histopathological studies were evaluated 24 h after PD administration. Results of the present study revealed that rats intoxicated with PD induced- oxidative stress and inflammation via an increase in malondialdehyde (MDA) and a decrease in nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway and glutathione(GSH) levels with an increase in brain contents of tumor necrosis factor-alpha (TNF-α) and interleukin (IL-6). Pre-treatment with TNG (100 mg/kg; orally) ameliorated behavior, cholinergic activities, and oxidative stress and decreased the elevated levels of pro-inflammatory mediators; TNF-α and IL-6 with a decrease in brain content of chromium residues detected by Plasma-Optical Emission Spectrometer. Also, the histopathological picture of the brain was improved significantly in rats that received TNG (100 mg/kg). Additionally, TNG decreased caspase-3 expression in the brain of PD rats. In conclusion, TNG possesses a significant neuroprotective role against PD-induced acute brain injury via modulating the Nrf2 signaling pathway and quenching the release of inflammatory mediators and apoptosis in rats.
Keywords: Brain Injury; Caspase-3; Chromium; Inflammation; Nrf2; Tangeretin.
© 2023. The Author(s).
Conflict of interest statement
Authors declare no conflict of interest.
Figures
Similar articles
-
L-carnitine and Co Q10 ameliorate potassium dichromate -induced acute brain injury in rats targeting AMPK/AKT/NF-κβ.Int Immunopharmacol. 2021 Dec;101(Pt B):107867. doi: 10.1016/j.intimp.2021.107867. Epub 2021 Sep 4. Int Immunopharmacol. 2021. PMID: 34489184
-
Vinpocetine ameliorates L-arginine induced acute pancreatitis via Sirt1/Nrf2/TNF pathway and inhibition of oxidative stress, inflammation, and apoptosis.Biomed Pharmacother. 2021 Jan;133:110976. doi: 10.1016/j.biopha.2020.110976. Epub 2020 Nov 14. Biomed Pharmacother. 2021. Retraction in: Biomed Pharmacother. 2024 Jan;170:116045. doi: 10.1016/j.biopha.2023.116045. PMID: 33202281 Retracted.
-
Neuroprotective effect of resveratrol against radiation after surgically induced brain injury by reducing oxidative stress, inflammation, and apoptosis through NRf2/HO-1/NF-κB signaling pathway.J Biochem Mol Toxicol. 2020 Dec;34(12):e22600. doi: 10.1002/jbt.22600. Epub 2020 Aug 20. J Biochem Mol Toxicol. 2020. PMID: 32815255
-
Febuxostat attenuates aluminum chloride-induced hepatorenal injury in rats with the impact of Nrf2, Crat, Car3, and MNK-mediated apoptosis.Environ Sci Pollut Res Int. 2023 Jul;30(35):83356-83375. doi: 10.1007/s11356-023-28182-9. Epub 2023 Jun 20. Environ Sci Pollut Res Int. 2023. PMID: 37340161 Free PMC article.
-
Appraisal of the Pre-Emptive Effect of Lactoferrin Against Chromium-Induced Testicular Toxicity in Male Rats.Biol Trace Elem Res. 2023 Nov;201(11):5321-5334. doi: 10.1007/s12011-023-03605-3. Epub 2023 Mar 6. Biol Trace Elem Res. 2023. PMID: 36877398 Free PMC article.
Cited by
-
The Neuroprotective Role of Tangeritin.CNS Neurol Disord Drug Targets. 2025;24(2):144-157. doi: 10.2174/0118715273325789240904065214. CNS Neurol Disord Drug Targets. 2025. PMID: 39297465 Review.
-
An Update on the Potential of Tangeretin in the Management of Neuroinflammation-Mediated Neurodegenerative Disorders.Life (Basel). 2024 Apr 14;14(4):504. doi: 10.3390/life14040504. Life (Basel). 2024. PMID: 38672774 Free PMC article. Review.
-
Tangeretin attenuates bleomycin-induced pulmonary fibrosis by inhibiting epithelial-mesenchymal transition via the PI3K/Akt pathway.Front Pharmacol. 2023 Sep 15;14:1247800. doi: 10.3389/fphar.2023.1247800. eCollection 2023. Front Pharmacol. 2023. PMID: 37781713 Free PMC article.
-
Synergistic effect of arginine and Lactobacillus plantarum against potassium dichromate induced-acute liver and kidney injury in rats: Role of iNOS and TLR4/NF-κB signaling pathways.Iran J Basic Med Sci. 2023;26(8):941-952. doi: 10.22038/IJBMS.2023.68855.15108. Iran J Basic Med Sci. 2023. PMID: 37427328 Free PMC article.
-
Tangeretin inhibits airway inflammatory responses by reducing early growth response 1 (EGR1) expression in mice exposed to cigarette smoke and lipopolysaccharide.Heliyon. 2024 Oct 24;10(21):e39797. doi: 10.1016/j.heliyon.2024.e39797. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39553588 Free PMC article.
References
-
- Abu Zeid EH, Hussein MMA, Ali H. Ascorbic acid protects male rat brain from oral potassium dichromate-induced oxidative DNA damage and apoptotic changes: the expression patterns of caspase-3, P 53, Bax, and Bcl-2 genes. Environ Sci Pollut Res. 2018;25(13):13056–13066. doi: 10.1007/s11356-018-1546-9. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
