Dabrafenib, alone or in combination with trametinib, in BRAF V600-mutated pediatric Langerhans cell histiocytosis
- PMID: 36884302
- PMCID: PMC10393756
- DOI: 10.1182/bloodadvances.2022008414
Dabrafenib, alone or in combination with trametinib, in BRAF V600-mutated pediatric Langerhans cell histiocytosis
Abstract
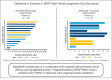
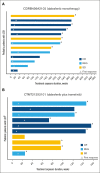
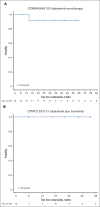
Langerhans cell histiocytosis (LCH) is a rare, heterogenous, neoplastic disorder primarily affecting children. BRAF mutations have been reported in >50% of patients with LCH. The selective BRAF inhibitor, dabrafenib, in combination with the MEK1/2 inhibitor, trametinib, has been approved in select BRAF V600-mutant solid tumors. Two open-label phase 1/2 studies were conducted in pediatric patients with BRAF V600-mutant, recurrent/refractory malignancies treated with dabrafenib monotherapy (CDRB436A2102; NCT01677741) or dabrafenib plus trametinib (CTMT212X2101; NCT02124772). The primary objectives of both studies were to determine safe and tolerable doses that achieve similar exposure to the approved doses for adults. Secondary objectives included safety, tolerability, and preliminary antitumor activity. Thirteen and 12 patients with BRAF V600-mutant LCH received dabrafenib monotherapy and in combination with trametinib, respectively. Investigator-assessed objective response rates per Histiocyte Society criteria were 76.9% (95% confidence interval [CI], 46.2-95.0) and 58.3% (95% CI, 27.7-84.8) in the monotherapy and combination studies, respectively. More than 90% of responses were ongoing at study completion. The most common treatment-related adverse events (AEs) were vomiting and increased blood creatinine with monotherapy and pyrexia, diarrhea, dry skin, decreased neutrophil count, and vomiting with combination therapy. Two patients each discontinued treatment with monotherapy and combination therapy because of AEs. Overall, dabrafenib monotherapy or in combination with trametinib demonstrated clinical efficacy and manageable toxicity in relapsed/refractory BRAF V600-mutant pediatric LCH, with most responses ongoing. Safety was consistent with that reported in other pediatric and adult conditions treated with dabrafenib plus trametinib.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.A.W. reports research funding (to institution) from Novartis, consulting fees/participation in a data safety monitoring board with Pfizer, and consulting fees/participation in advisory boards for Jazz Pharmaceuticals. I.J.D. reports contracts (with institution) from Bristol Myers Squibb, Genentech, and Novartis; payment (to institution) for role as chair of the Pediatric Brain Tumor Consortium; consulting fees/participation in a data safety monitoring board with Celgene/Bristol Myers Squibb; and consulting fees/participation in advisory boards for AstraZeneca, Bristol Myers Squibb, Day One, Fennec, QED, and Roche. J.C. and L.O. report employment with Novartis. M. Russo and M. Roughton report stock/shares in and employment with Novartis. D.H. reports consulting fees from Novartis and participation in a data safety monitoring or advisory board for activities relating to trametinib and dabrafenib. B.G. declares no competing financial interests.
Figures
References
-
- Sterlich K, Minkov M. In: Rare Diseases - Diagnostic and Therapeutic Odyssey. Valarmathi MT, editor. 2021. Childhood Langerhans cell histiocytosis: epidemiology, clinical presentations, prognostic factors, and therapeutic approaches.
-
- Stalemark H, Laurencikas E, Karis J, Gavhed D, Fadeel B, Henter JI. Incidence of Langerhans cell histiocytosis in children: a population-based study. Pediatr Blood Cancer. 2008;51(1):76–81. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

