A non-hallucinogenic LSD analog with therapeutic potential for mood disorders
- PMID: 36884348
- PMCID: PMC10112881
- DOI: 10.1016/j.celrep.2023.112203
A non-hallucinogenic LSD analog with therapeutic potential for mood disorders
Abstract
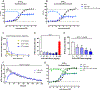
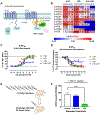
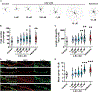
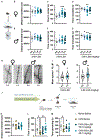
Hallucinations limit widespread therapeutic use of psychedelics as rapidly acting antidepressants. Here we profiled the non-hallucinogenic lysergic acid diethylamide (LSD) analog 2-bromo-LSD (2-Br-LSD) at more than 33 aminergic G protein-coupled receptors (GPCRs). 2-Br-LSD shows partial agonism at several aminergic GPCRs, including 5-HT2A, and does not induce the head-twitch response (HTR) in mice, supporting its classification as a non-hallucinogenic 5-HT2A partial agonist. Unlike LSD, 2-Br-LSD lacks 5-HT2B agonism, an effect linked to cardiac valvulopathy. Additionally, 2-Br-LSD produces weak 5-HT2A β-arrestin recruitment and internalization in vitro and does not induce tolerance in vivo after repeated administration. 2-Br-LSD induces dendritogenesis and spinogenesis in cultured rat cortical neurons and increases active coping behavior in mice, an effect blocked by the 5-HT2A-selective antagonist volinanserin (M100907). 2-Br-LSD also reverses the behavioral effects of chronic stress. Overall, 2-Br-LSD has an improved pharmacological profile compared with LSD and may have profound therapeutic value for mood disorders and other indications.
Keywords: 5-HT2A; 5-HT2B; CP: Molecular biology; CP: Neuroscience; G protein-coupled receptor; depression; hallucinogen; neuroplasticity; psychedelic; serotonin; stress; tolerance.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.G. and H.S. are employees of BetterLife Pharma Inc.
Figures



References
-
- Kessler RC, Sampson NA, Berglund P, Gruber MJ, Al-Hamzawi A, Andrade L, Bunting B, Demyttenaere K, Florescu S, de Girolamo G, et al. (2015). Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci 24, 210–226. 10.1017/S2045796015000189. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

