Immunogenicity and safety of concomitant and sequential administration of yellow fever YF-17D vaccine and tetravalent dengue vaccine candidate TAK-003: A phase 3 randomized, controlled study
- PMID: 36888687
- PMCID: PMC9994689
- DOI: 10.1371/journal.pntd.0011124
Immunogenicity and safety of concomitant and sequential administration of yellow fever YF-17D vaccine and tetravalent dengue vaccine candidate TAK-003: A phase 3 randomized, controlled study
Abstract
Background: Yellow fever (YF) vaccination is often mandatory for travelers to YF-endemic areas. The areas with risk of YF partially overlap with those of dengue, for which there is currently no recommended vaccine available for dengue-naïve individuals. This phase 3 study assessed the immunogenicity and safety of concomitant and sequential administration of YF (YF-17D) and tetravalent dengue (TAK-003) vaccines in healthy adults aged 18-60 years living in areas of the US non-endemic for either virus.
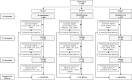
Methods: Participants were randomized 1:1:1 to receive the following vaccinations at Months 0, 3, and 6, respectively: YF-17D+placebo, TAK-003, and TAK-003 (Group 1); TAK-003+placebo, TAK-003, and YF-17D (Group 2); or YF-17D+TAK-003, TAK-003, and placebo (Group 3). The primary objective was to demonstrate non-inferiority (upper bound of 95% confidence interval [UB95%CI] of difference <5%) of YF seroprotection rate one month following concomitant administration of YF-17D and TAK-003 (Group 3) compared with YF-17D plus placebo (Group 1). The secondary objectives included demonstration of non-inferiority of YF and dengue geometric mean titers (GMTs) (UB95%CI for GMT ratio <2.0), and safety.
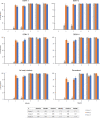
Results: 900 adults were randomized. YF seroprotection rates one month post-YF-17D (Month 1) were 99.5% and 99.1% in Group 1 and 3, respectively, and non-inferiority was demonstrated (UB95%CI = 2.69% i.e. <5%). Non-inferiority was also demonstrated for GMTs against YF one month post-YF-17D, and against DENV-2, -3, and -4 (UB95%CI <2), but not DENV-1 (UB95%CI: 2.22), one month post-second TAK-003 vaccination. Adverse event rates following TAK-003 were consistent with previous results, and no important safety risks were identified.
Conclusions: In this study, YF-17D vaccine and TAK-003 were immunogenic and well tolerated when sequentially or concomitantly administered. The non-inferiority of immune responses to YF-17D and TAK-003 was demonstrated for concomitant administration of the 2 vaccines compared to separate vaccination, except against DENV-1 but with GMTs similar to those observed in other TAK-003 trials.
Trial registration: ClinicalTrials.gov identified: NCT03342898.
Copyright: © 2023 Tricou et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: VT, IE, MR, MB, IL and DW are full-time employees of Takeda. AB was employee of Takeda at the time of the study (current affiliation of AB: HilleVax GmbH, Zurich, Switzerland). BE, JEE, and MT received funding from Takeda Vaccines to perform the trial.
Figures


References
-
- World Health Organization. Ten threats to global health in 2019 [6 April 2020]. Available from: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-....
-
- World Health Organization. Yellow fever fact sheet 2019 [cited 29 April 2020]. Available from: https://www.who.int/news-room/fact-sheets/detail/yellow-fever.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

