Simple monocyclic pyrimidine analogs as microtubule targeting agents binding to the colchicine site
- PMID: 36889150
- PMCID: PMC10084637
- DOI: 10.1016/j.bmc.2023.117217
Simple monocyclic pyrimidine analogs as microtubule targeting agents binding to the colchicine site
Abstract
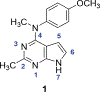
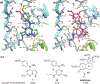
Complex natural products that bind to tubulin/microtubules come under the broad category of microtubule binding agents. The design of simplified analogs of previously reported bicyclic, microtubule depolymerizer, pyrrolo[2,3-d]pyrimidine, provided valuable structure-activity relationship data and led to the identification of novel monocyclic pyrimidine analogs of which 12 was 47-fold more potent (EC50 123 nM) for cellular microtubule depolymerization activity and 7.5-fold more potent (IC50 24.4 nM) at inhibiting the growth of MDA-MB-435 cancer cells, suggesting significantly better binding of the target within the colchicine site of tubulin compared to lead compound 1. This compound and others of this series of monocyclic pyrimidine analogs were able to overcome multidrug resistance due to the expression of the βIII-isotype of tubulin and P-glycoprotein. In vivo evaluation of the most potent analog 12 in an MDA-MB-435 xenograft mouse model indicated, along with paclitaxel, that both compounds showed a trend towards lower tumor volume however neither compound showed significant antitumor activity in the trial. To our knowledge these are the first examples of simple substituted monocyclic pyrimidines as colchicine site binding antitubulin compounds with potent antitumor activity.
Keywords: Colchicine site agents; Cytotoxins; Microtubule targeting agents; Pyrimidines.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



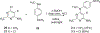


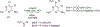

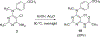
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

