Estradiol regulates leptin sensitivity to control feeding via hypothalamic Cited1
- PMID: 36889283
- PMCID: PMC10028007
- DOI: 10.1016/j.cmet.2023.02.004
Estradiol regulates leptin sensitivity to control feeding via hypothalamic Cited1
Abstract
Until menopause, women have a lower propensity to develop metabolic diseases than men, suggestive of a protective role for sex hormones. Although a functional synergy between central actions of estrogens and leptin has been demonstrated to protect against metabolic disturbances, the underlying cellular and molecular mechanisms mediating this crosstalk have remained elusive. By using a series of embryonic, adult-onset, and tissue/cell-specific loss-of-function mouse models, we document an unprecedented role of hypothalamic Cbp/P300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 1 (Cited1) in mediating estradiol (E2)-dependent leptin actions that control feeding specifically in pro-opiomelanocortin (Pomc) neurons. We reveal that within arcuate Pomc neurons, Cited1 drives leptin's anorectic effects by acting as a co-factor converging E2 and leptin signaling via direct Cited1-ERα-Stat3 interactions. Together, these results provide new insights on how melanocortin neurons integrate endocrine inputs from gonadal and adipose axes via Cited1, thereby contributing to the sexual dimorphism in diet-induced obesity.
Keywords: ARC; Pomc; diet-induced obesity; estradiol; hypothalamus; leptin.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.H.T. is a member of the scientific advisory board of ERX Pharmaceuticals, Inc., Cambridge, MA. He was a member of the Research Cluster Advisory Panel (ReCAP) of the Novo Nordisk Foundation between 2017 and 2019. He attended a scientific advisory board meeting of the Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, in 2016. He received funding for his research projects by Novo Nordisk (2016–2020) and Sanofi-Aventis (2012–2019). He consulted for Böhringer Ingelheim Pharma GmbH & Co. KG (2020). He delivered a scientific lecture for Sanofi-Aventis Deutschland GmbH in 2020. He is an editorial board member of Cell Metabolism. He confirms that to the best of his knowledge none of the above were involved in the preparation of this paper.
Figures

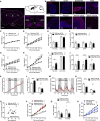

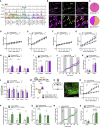

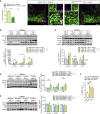

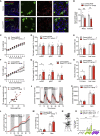
Comment in
-
CITED1 mediates sex-specific regulation of food intake in hypothalamic neurons.Nat Rev Endocrinol. 2023 May;19(5):253. doi: 10.1038/s41574-023-00829-0. Nat Rev Endocrinol. 2023. PMID: 36949099 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

