Organ-specific fuel rewiring in acute and chronic hypoxia redistributes glucose and fatty acid metabolism
- PMID: 36889284
- PMCID: PMC10077660
- DOI: 10.1016/j.cmet.2023.02.007
Organ-specific fuel rewiring in acute and chronic hypoxia redistributes glucose and fatty acid metabolism
Abstract
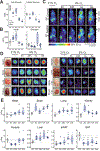
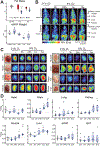
Oxygen deprivation can be detrimental. However, chronic hypoxia is also associated with decreased incidence of metabolic syndrome and cardiovascular disease in high-altitude populations. Previously, hypoxic fuel rewiring has primarily been studied in immortalized cells. Here, we describe how systemic hypoxia rewires fuel metabolism to optimize whole-body adaptation. Acclimatization to hypoxia coincided with dramatically lower blood glucose and adiposity. Using in vivo fuel uptake and flux measurements, we found that organs partitioned fuels differently during hypoxia adaption. Acutely, most organs increased glucose uptake and suppressed aerobic glucose oxidation, consistent with previous in vitro investigations. In contrast, brown adipose tissue and skeletal muscle became "glucose savers," suppressing glucose uptake by 3-5-fold. Interestingly, chronic hypoxia produced distinct patterns: the heart relied increasingly on glucose oxidation, and unexpectedly, the brain, kidney, and liver increased fatty acid uptake and oxidation. Hypoxia-induced metabolic plasticity carries therapeutic implications for chronic metabolic diseases and acute hypoxic injuries.
Keywords: PET scan; TCA cycle; fatty acid metabolism; fuel rewiring; fuel uptake; glucose metabolism; hypoxia; isotope tracing; organ-specific metabolism.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests I.H.J. is a consultant for Maze Therapeutics and has a patent related to hypoxia therapy for mitochondrial disorders.
Figures



Comment in
-
Unanticipated metabolic plasticity in response to chronic hypoxia.Cell Metab. 2023 Mar 7;35(3):381-383. doi: 10.1016/j.cmet.2023.02.011. Cell Metab. 2023. PMID: 36889278
References
-
- Lankford AR, Yang J-N, Rose’Meyer R, French BA, Matherne GP, Fredholm BB, and Yang Z (2006). Effect of modulating cardiac A1 adenosine receptor expression on protection with ischemic preconditioning. American Journal of Physiology-Heart and Circulatory Physiology 290, H1469–H1473. 10.1152/ajpheart.00181.2005. - DOI - PubMed

