Gaucher Disease Types I and III Responded Well to Substrate Reduction Therapy Using Eliglustat
- PMID: 36889706
- PMCID: PMC10641180
- DOI: 10.2169/internalmedicine.1425-22
Gaucher Disease Types I and III Responded Well to Substrate Reduction Therapy Using Eliglustat
Abstract
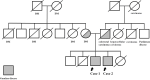
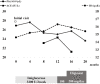
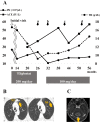
Gaucher disease (GD) causes the accumulation of glucocerebrosides in various organs, resulting in hepatosplenomegaly, anemia, decreased platelet counts, and bone disorders. Glucosylsphingosine accumulates in the brain and causes central nervous system (CNS) disorders. GD can be classified into types I (without CNS disorders), II, and III. Substrate reduction therapy (SRT) is an oral therapy that improves patients' quality of life; however, its effect on type III GD is unknown. We administered SRT to GD types I and III patients and found it effective. Malignancy is a late complication of GD, but this is the first report of Barrett adenocarcinoma.
Keywords: Gaucher disease; enzyme replacement therapy; glucocerebrosidase; glucocerebroside; glucosyl sphingosine; substrate reduction therapy.
Conflict of interest statement
Figures
Similar articles
-
Eliglustat tartrate for the treatment of adults with type 1 Gaucher disease.Drug Des Devel Ther. 2015 Aug 18;9:4639-47. doi: 10.2147/DDDT.S77760. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26345314 Free PMC article. Review.
-
Recommendations for the use of eliglustat in the treatment of adults with Gaucher disease type 1 in the United States.Mol Genet Metab. 2016 Feb;117(2):95-103. doi: 10.1016/j.ymgme.2015.09.002. Epub 2015 Sep 7. Mol Genet Metab. 2016. PMID: 26387627 Review.
-
Gaucher disease and its treatment options.Ann Pharmacother. 2013 Sep;47(9):1182-93. doi: 10.1177/1060028013500469. Ann Pharmacother. 2013. PMID: 24259734 Review.
-
Biochemical response to substrate reduction therapy versus enzyme replacement therapy in Gaucher disease type 1 patients.Orphanet J Rare Dis. 2016 Mar 24;11:28. doi: 10.1186/s13023-016-0413-3. Orphanet J Rare Dis. 2016. PMID: 27008851 Free PMC article.
-
Management and monitoring recommendations for the use of eliglustat in adults with type 1 Gaucher disease in Europe.Eur J Intern Med. 2017 Jan;37:25-32. doi: 10.1016/j.ejim.2016.07.011. Epub 2016 Aug 10. Eur J Intern Med. 2017. PMID: 27522145 Review.
References
-
- Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab 83: 6-15, 2004. - PubMed
-
- Nilsson O, Svennerholm L. Accumulation of glucosylceramide and glucosylsphingosine (psychosine) in cerebrum and cerebellum in infantile and juvenile Gaucher disease. J Neurochem 39: 709-718, 1982. - PubMed
-
- Lukina E, Watman N, Dragosky M, et al. . Eliglustat, an investigational oral therapy for Gaucher disease type 1: Phase 2 trial results after 4 years of treatment. Blood Cells Mol Dis 53: 274-276, 2014. - PubMed
-
- Cox TM, Drelichman G, Cravo R, et al. . Eliglustat compared with imiglucerase in patients with Gaucher's disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomized, open-label, non-inferiority trial. Lancet 385: 2355-2362, 2015. - PubMed
-
- The International Collaborative Gaucher Group. Gaucher Registry 2010 Annual Report (Data cutoff 31 Dec. 2009). Gen-zyme 2010.

