Gaucher Disease Types I and III Responded Well to Substrate Reduction Therapy Using Eliglustat
- PMID: 36889706
- PMCID: PMC10641180
- DOI: 10.2169/internalmedicine.1425-22
Gaucher Disease Types I and III Responded Well to Substrate Reduction Therapy Using Eliglustat
Abstract
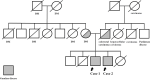
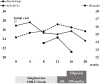
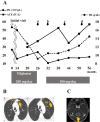
Gaucher disease (GD) causes the accumulation of glucocerebrosides in various organs, resulting in hepatosplenomegaly, anemia, decreased platelet counts, and bone disorders. Glucosylsphingosine accumulates in the brain and causes central nervous system (CNS) disorders. GD can be classified into types I (without CNS disorders), II, and III. Substrate reduction therapy (SRT) is an oral therapy that improves patients' quality of life; however, its effect on type III GD is unknown. We administered SRT to GD types I and III patients and found it effective. Malignancy is a late complication of GD, but this is the first report of Barrett adenocarcinoma.
Keywords: Gaucher disease; enzyme replacement therapy; glucocerebrosidase; glucocerebroside; glucosyl sphingosine; substrate reduction therapy.
Conflict of interest statement
Figures
References
-
- Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab 83: 6-15, 2004. - PubMed
-
- Nilsson O, Svennerholm L. Accumulation of glucosylceramide and glucosylsphingosine (psychosine) in cerebrum and cerebellum in infantile and juvenile Gaucher disease. J Neurochem 39: 709-718, 1982. - PubMed
-
- Lukina E, Watman N, Dragosky M, et al. Eliglustat, an investigational oral therapy for Gaucher disease type 1: Phase 2 trial results after 4 years of treatment. Blood Cells Mol Dis 53: 274-276, 2014. - PubMed
-
- Cox TM, Drelichman G, Cravo R, et al. Eliglustat compared with imiglucerase in patients with Gaucher's disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomized, open-label, non-inferiority trial. Lancet 385: 2355-2362, 2015. - PubMed
-
- The International Collaborative Gaucher Group. Gaucher Registry 2010 Annual Report (Data cutoff 31 Dec. 2009). Gen-zyme 2010.

