Inhibitory effect of 405 nm laser light on bacterial biofilm in urethral stent
- PMID: 36890147
- PMCID: PMC9995349
- DOI: 10.1038/s41598-023-30280-0
Inhibitory effect of 405 nm laser light on bacterial biofilm in urethral stent
Abstract
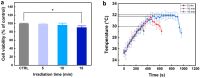
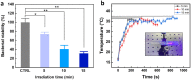
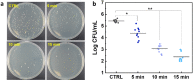
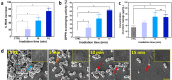
The clinical use of urethral stents is usually complicated by various adverse effects, including dysuria, fever, and urinary tract infection (UTI). Biofilms (formed by bacteria, such as Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus) adhering to the stent cause UTIs in stented patients (approximately 11%). The undesirable consequences of antibiotics use include bacterial resistance, weight gain, and type 1 diabetes, which occur when antibiotics are used for a long time. We aimed to assess the efficacy of a new optical treatment with a 405 nm laser to inhibit bacterial growth in a urethral stent in vitro. The urethral stent was grown in S. aureus broth media for three days to induce biofilm formation under dynamic conditions. Various irradiation times with the 405 nm laser light were tested (5, 10, and 15 min). The efficacy of the optical treatment on biofilms was evaluated quantitatively and qualitatively. The production of reactive oxygen species helped eliminate the biofilm over the urethral stent after 405 nm irradiation. The inhibition rate corresponded to a 2.2 log reduction of colony-forming units/mL of bacteria after 0.3 W/cm2 of irradiation for 10 min. The treated stent showed a significant reduction in biofilm formation compared with the untreated stent, as demonstrated by SYTO 9 and propidium iodide staining. MTT assays using the CCD-986sk cell line revealed no toxicity after 10 min of irradiation. We conclude that optical treatment with 405 nm laser light inhibits bacterial growth in urethral stents with no or minimal toxicity.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Pseudomonas aeruginosa Increases the Sensitivity of Biofilm-Grown Staphylococcus aureus to Membrane-Targeting Antiseptics and Antibiotics.mBio. 2019 Jul 30;10(4):e01501-19. doi: 10.1128/mBio.01501-19. mBio. 2019. PMID: 31363032 Free PMC article.
-
Characterization of Blue Light Treatment for Infected Wounds: Antibacterial Efficacy of 420, 455, and 480 nm Light-Emitting Diode Arrays Against Common Skin Pathogens Versus Blue Light-Induced Skin Cell Toxicity.Photobiomodul Photomed Laser Surg. 2021 May;39(5):339-348. doi: 10.1089/photob.2020.4932. Photobiomodul Photomed Laser Surg. 2021. PMID: 33961502
-
Prevention of initial biofilm formation on ureteral stents using a sustained releasing varnish containing chlorhexidine: in vitro study.J Endourol. 2013 Mar;27(3):333-7. doi: 10.1089/end.2012.0193. Epub 2012 Dec 6. J Endourol. 2013. PMID: 22970837
-
Melittin and its potential in the destruction and inhibition of the biofilm formation by Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa isolated from bovine milk.Microb Pathog. 2017 Nov;112:57-62. doi: 10.1016/j.micpath.2017.09.046. Epub 2017 Sep 21. Microb Pathog. 2017. PMID: 28943153
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
References
-
- Duvdevani M, Chew BH, Denstedt JD. Urethral stents: Review of technology and clinical applications. Interv. Manag. Urol. Dis. 2006;8:191–206.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

