Treatment options for patients with human epidermal growth factor 2-positive breast cancer brain metastases: A systematic review and meta-analysis
- PMID: 36890831
- PMCID: PMC9986525
- DOI: 10.3389/fonc.2023.1003565
Treatment options for patients with human epidermal growth factor 2-positive breast cancer brain metastases: A systematic review and meta-analysis
Abstract
Introduction: Many systemic treatment options are available for patients with human epidermal growth factor 2 (HER2)-positive breast cancer brain metastases. However, it is unclear which pharmacological treatment option is the most beneficial.
Methods: We searched databases, such as PubMed, Embase, and Cochrane Library, and conference abstracts according to keywords. We extracted progression-free survival (PFS), overall survival (OS) data, and overall response rate (ORR) from randomized controlled trials and single-arm studies of HER2-positive breast cancer brain metastasis treatment for meta-analysis and analyzed different drug-related adverse events (AEs).
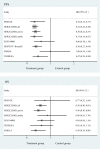
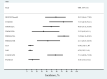
Results: Three randomized controlled trials and seven single-arm clinical studies with 731 patients with HER2-positive brain metastases from breast cancer involving at least seven drugs were included. In randomized controlled trials, our results showed that trastuzumab deruxtecan significantly improved PFS and OS in patients and was superior to other drug regimens. In the single-arm study, the ORR was more pronounced for the trastuzumab deruxtecan and pyrotinib plus capecitabine regimens (ORR, 73.33%; 95% confidence intervals [CI], 44.90%-92.21%; ORR, 74.58%; 95% CI, 61.56%-85.02%, respectively). We found that the main AEs of antibody-drug conjugate (ADC) were nausea and fatigue, while the main AE of small-molecule tyrosine kinase inhibitor (TKI) drugs and large monoclonal antibodies was diarrhea.
Conclusions: Trastuzumab deruxtecan was shown to be the most significant in improving survival in patients with HER2-positive breast cancer brain metastases in network meta-analysis, and in single-arm study, patients with HER2-positive breast cancer brain metastases treated with trastuzumab deruxtecan and pyrotinib plus capecitabine regimen had the highest ORR. The main AEs associated with ADC, large monoclonal antibodies, and TKI drugs were nausea, fatigue, and diarrhea, respectively.
Keywords: HER2; brain metastases; breast cancer; meta-analysis; treatment.
Copyright © 2023 Huo, Shen, Wang, Li, Xie, Liu, Wang, Zhao, Ren and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. histology, multiplicity, surgery, and survival. Cancer (1996) 78:1781–8. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

