Human EGFRvIII chimeric antigen receptor T cells demonstrate favorable safety profile and curative responses in orthotopic glioblastoma
- PMID: 36890859
- PMCID: PMC9986233
- DOI: 10.1002/cti2.1440
Human EGFRvIII chimeric antigen receptor T cells demonstrate favorable safety profile and curative responses in orthotopic glioblastoma
Abstract
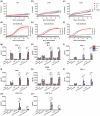
Objectives: Glioblastoma is a highly aggressive and fatal brain malignancy, and effective targeted therapies are required. The combination of standard treatments including surgery, chemotherapy and radiotherapy is not curative. Chimeric antigen receptor (CAR) T cells are known to cross the blood-brain barrier, mediating antitumor responses. A tumor-expressed deletion mutant of the epidermal growth factor receptor (EGFRvIII) is a robust CAR T cell target in glioblastoma. Here, we show our de novo generated, high-affinity EGFRvIII-specific CAR; GCT02, demonstrating curative efficacy in human orthotopic glioblastoma models.
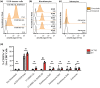
Methods: The GCT02 binding epitope was predicted using Deep Mutational Scanning (DMS). GCT02 CAR T cell cytotoxicity was investigated in three glioblastoma models in vitro using the IncuCyte platform, and cytokine secretion with a cytometric bead array. GCT02 in vivo functionality was demonstrated in two NSG orthotopic glioblastoma models. The specificity profile was generated by measuring T cell degranulation in response to coculture with primary human healthy cells.
Results: The GCT02 binding location was predicted to be located at a shared region of EGFR and EGFRvIII; however, the in vitro functionality remained exquisitely EGFRvIII specific. A single CAR T cell infusion generated curative responses in two orthotopic models of human glioblastoma in NSG mice. The safety analysis further validated the specificity of GCT02 for mutant-expressing cells.
Conclusion: This study demonstrates the preclinical functionality of a highly specific CAR targeting EGFRvIII on human cells. This CAR could be an effective treatment for glioblastoma and warrants future clinical investigation.
Keywords: CAR T cells; EGFRvIII; chimeric antigen receptor; glioblastoma; immunotherapy.
© 2023 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
RCA, RSC and MRJ are listed as inventors on a patent filed with the GCT02 CAR presented here.
Figures




References
-
- Miller KD, Ostrom QT, Kruchko C et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin 2021; 71: 381–406. - PubMed
-
- Brain and Other Central Nervous System Cancers. Canberra: Australian Institute of Health and Welfare; 2017:80.
-
- Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987–996. - PubMed
-
- van Tellingen O, Yetkin‐Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat 2015; 19: 1–12. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
