CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies
- PMID: 36891310
- PMCID: PMC9986336
- DOI: 10.3389/fimmu.2023.1101495
CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies
Abstract
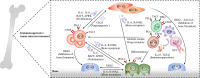
Over the last decade, the survival outcome of patients with multiple myeloma (MM) has been substantially improved with the emergence of novel therapeutic agents, such as proteasome inhibitors, immunomodulatory drugs, anti-CD38 monoclonal antibodies, selective inhibitors of nuclear export (SINEs), and T cell redirecting bispecific antibodies. However, MM remains an incurable neoplastic plasma cell disorder, and almost all MM patients inevitably relapse due to drug resistance. Encouragingly, B cell maturation antigen (BCMA)-targeted chimeric antigen receptor T (CAR-T) cell therapy has achieved impressive success in the treatment of relapsed/refractory (R/R) MM and brought new hopes for R/R MM patients in recent years. Due to antigen escape, the poor persistence of CAR-T cells, and the complicated tumor microenvironment, a significant population of MM patients still experience relapse after anti-BCMA CAR-T cell therapy. Additionally, the high manufacturing costs and time-consuming manufacturing processes caused by the personalized manufacturing procedures also limit the broad clinical application of CAR-T cell therapy. Therefore, in this review, we discuss current limitations of CAR-T cell therapy in MM, such as the resistance to CAR-T cell therapy and the limited accessibility of CAR-T cell therapy, and summarize some optimization strategies to overcome these challenges, including optimizing CAR structure, such as utilizing dual-targeted/multi-targeted CAR-T cells and armored CAR-T cells, optimizing manufacturing processes, combing CAR-T cell therapy with existing or emerging therapeutic approaches, and performing subsequent anti-myeloma therapy after CAR-T cell therapy as salvage therapy or maintenance/consolidation therapy.
Keywords: CAR-T cell exhaustion; CAR-T cell therapy; antigen escape; combinatorial therapy; immunosuppressive tumor microenvironment; relapse.
Copyright © 2023 Zhang, Zhang, Lan, Wu and Xiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Misund K, Hofste Op Bruinink D, Coward E, Hoogenboezem RM, Rustad EH, Sanders MA, et al. Clonal evolution after treatment pressure in multiple myeloma: Heterogenous genomic aberrations and transcriptomic convergence. Leukemia (2022) 36(7):1887–97. doi: 10.1038/s41375-022-01597-y - DOI - PMC - PubMed
-
- Zhao WH, Liu J, Wang BY, Chen YX, Cao XM, Yang Y, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against b cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol (2018) 11(1):141. doi: 10.1186/s13045-018-0681-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

