Cutaneous manifestations associated with immune checkpoint inhibitors
- PMID: 36891313
- PMCID: PMC9986601
- DOI: 10.3389/fimmu.2023.1071983
Cutaneous manifestations associated with immune checkpoint inhibitors
Abstract
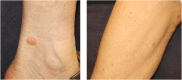
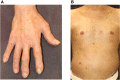
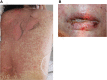
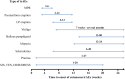
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that block key mediators of tumor-mediated immune evasion. The frequency of its use has increased rapidly and has extended to numerous cancers. ICIs target immune checkpoint molecules, such as programmed cell death protein 1 (PD-1), PD ligand 1 (PD-L1), and T cell activation, including cytotoxic T-lymphocyte-associated protein-4 (CTLA-4). However, ICI-driven alterations in the immune system can induce various immune-related adverse events (irAEs) that affect multiple organs. Among these, cutaneous irAEs are the most common and often the first to develop. Skin manifestations are characterized by a wide range of phenotypes, including maculopapular rash, psoriasiform eruption, lichen planus-like eruption, pruritus, vitiligo-like depigmentation, bullous diseases, alopecia, and Stevens-Johnson syndrome/toxic epidermal necrolysis. In terms of pathogenesis, the mechanism of cutaneous irAEs remains unclear. Still, several hypotheses have been proposed, including activation of T cells against common antigens in normal tissues and tumor cells, increased release of proinflammatory cytokines associated with immune-related effects in specific tissues/organs, association with specific human leukocyte antigen variants and organ-specific irAEs, and acceleration of concurrent medication-induced drug eruptions. Based on recent literature, this review provides an overview of each ICI-induced skin manifestation and epidemiology and focuses on the mechanisms underlying cutaneous irAEs.
Keywords: CTLA-4; PD-1; PD-L1; cutaneous manifestation; epidemiology; immune checkpoint inhibitors; immune-related adverse events.
Copyright © 2023 Watanabe and Yamaguchi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

