Interference in point-of-care international normalized ratio monitoring in patients with lupus anticoagulant is correlated with anti-β2-glycoprotein I antibody titers
- PMID: 36891523
- PMCID: PMC9986099
- DOI: 10.1016/j.rpth.2022.100011
Interference in point-of-care international normalized ratio monitoring in patients with lupus anticoagulant is correlated with anti-β2-glycoprotein I antibody titers
Abstract
Background: Patients with antiphospholipid syndrome (APS) receive anticoagulant therapy with vitamin K antagonists (VKAs) to prevent recurrent thrombosis. VKA treatment requires strict monitoring with an international normalized ratio (INR). It is known that lupus anticoagulants (LAs) can lead to elevated INR results with point-of-care-testing (POCT) devices, which could result in inadequate adaptation of anticoagulant therapy.
Objective: To determine discrepancies between POCT-INR and laboratory-INR in patients who are LA-positive on VKA therapy.
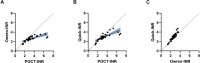
Methods: Paired INR testing was performed with 1 POCT device (CoaguChek XS) and 2 laboratory assays (Owren and Quick method) in 33 patients with LA-positive APS on VKA in a single-center cross-sectional study. Patients were tested for anti-β2-glycoprotein I, anticardiolipin, and antiphosphatidylserine/prothrombin immunoglobulin (Ig) G and IgM antibodies. Agreement between assays was evaluated with Spearman's correlation, Lin's correlation coefficient, and Bland-Altman plots. Agreement limits were considered satisfactory if differences were ≤20% as determined by the Clinical and Laboratory Standards Institute.
Results: We found poor agreement between POCT-INR and laboratory-INR based on Lin's concordance correlation coefficient (ρc) of 0.42 (95% CI, 0.26-0.55) between POCT-INR and Owren-INR, a ρc of 0.64 (95% CI, 0.47-0.76) between POCT-INR and Quick-INR, and a ρc of 0.77 (95% CI, 0.64-0.85) between Quick-INR and Owren-INR. High anti-β2-glycoprotein I IgG antibody titers correlated with INR disagreement between POCT-INR and laboratory-INR.
Conclusion: There is a disagreement between INR values measured with the CoaguChek XS and laboratory-INR in a proportion of patients with LA. Consequently, laboratory-INR monitoring should be preferred over POCT-INR monitoring in patients with LA-positive APS, especially in patients with high anti-β2-glycoprotein IgG antibody titers.
Keywords: anticoagulants; international normalized ratio; lupus coagulation inhibitor; point-of-care testing; warfarin.
© 2022 The Authors.
Figures

References
-
- Keeling D., Mackie I., Moore G.W., Greer I.A., Greaves M. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157:47–58. - PubMed
-
- Limper M., de Leeuw K., Lely A.T., Westerink J., Teng Y.K.O., Eikenboom J., et al. Diagnosing and treating antiphospholipid syndrome: a consensus paper. Neth J Med. 2019;77:98–108. - PubMed
-
- Miyakis S., Lockshin M.D., Atsumi T., Branch D.W., Brey R.L., Cervera R., et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. - PubMed
-
- Pengo V., Banzato A., Denas G., Jose S.P., Bison E., Hoxha A., et al. Correct laboratory approach to APS diagnosis and monitoring. Autoimmun Rev. 2013;12:832–834. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

