Myocardial steatosis impairs left ventricular diastolic-systolic coupling in healthy humans
- PMID: 36891609
- PMCID: PMC10318480
- DOI: 10.1113/JP284272
Myocardial steatosis impairs left ventricular diastolic-systolic coupling in healthy humans
Abstract
Mounting evidence suggests that myocardial steatosis contributes to left ventricular diastolic dysfunction, but definitive evidence in humans is lacking due to confounding comorbidities. As such, we utilized a 48-h food restriction model to acutely increase myocardial triglyceride (mTG) content - measured by 1 H magnetic resonance spectroscopy - in 27 young healthy volunteers (13 men/14 women). Forty-eight hours of fasting caused a more than 3-fold increase in mTG content (P < 0.001). Diastolic function - defined as early diastolic circumferential strain rate (CSRd) - was unchanged following the 48-h fasting intervention, but systolic circumferential strain rate was elevated (P < 0.001), indicative of systolic-diastolic uncoupling. Indeed, in a separate control experiment in 10 individuals, administration of low-dose dobutamine (2 μg/kg/min) caused a similar change in systolic circumferential strain rate as was found during 48 h of food restriction, along with a proportionate increase in CSRd, such that the two metrics remained coupled. Taken together, these data indicate that myocardial steatosis contributes to diastolic dysfunction by impairing diastolic-systolic coupling in healthy adults, and suggest that steatosis may contribute to the progression of heart disease. KEY POINTS: Preclinical evidence strongly suggests that myocardial lipid accumulation (termed steatosis) is an important mechanism driving heart disease. Definitive evidence in humans is limited due to the confounding influence of multiple underlying comorbidities. Using a 48-h food restriction model to acutely increase myocardial triglyceride content in young healthy volunteers, we demonstrate an association between myocardial steatosis and left ventricular diastolic dysfunction. These data advance the hypothesis that myocardial steatosis may contribute to diastolic dysfunction and suggest myocardial steatosis as a putative therapeutic target.
Keywords: coupling; diastole; lipotoxicity; relaxation; steatosis.
© 2023 The Authors. The Journal of Physiology © 2023 The Physiological Society.
Conflict of interest statement
Conflict of Interest
None declared.
Figures


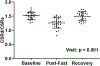

References
-
- Bakermans AJ, Geraedts TR, van Weeghel M, Denis S, Joao Ferraz M, Aerts JM, Aten J, Nicolay K, Houten SM & Prompers JJ. (2011). Fasting-induced myocardial lipid accumulation in long-chain acyl-CoA dehydrogenase knockout mice is accompanied by impaired left ventricular function. Circ Cardiovasc Imaging 4, 558–565. - PubMed
-
- Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS & Schaffer JE. (2005). Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res 96, 225–233. - PubMed
-
- Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB & Nielsen LB. (2003). Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology 144, 3483–3490. - PubMed
-
- Di Paola M, Cocco T & Lorusso M. (2000). Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry 39, 6660–6668. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

