Renal Protection of Mineralocorticoid Receptor Antagonist, Finerenone, in Diabetic Kidney Disease
- PMID: 36891650
- PMCID: PMC10008664
- DOI: 10.3803/EnM.2022.1629
Renal Protection of Mineralocorticoid Receptor Antagonist, Finerenone, in Diabetic Kidney Disease
Abstract
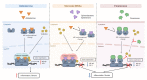
Chronic kidney disease (CKD) is the most common cause of end-stage renal disease in patients with type 2 diabetes mellitus (T2DM). CKD increases the risk of cardiovascular diseases; therefore, its prevention and treatment are important. The prevention of diabetic kidney disease (DKD) can be achieved through intensive glycemic control and blood pressure management. Additionally, DKD treatment aims to reduce albuminuria and improve kidney function. In patients with T2DM, renin-angiotensin-aldosterone system inhibitors, sodium glucose cotransporter 2 inhibitors, and glucagon-like peptide-1 receptor agonists can delay the progression of DKD. Hence, there is a need for novel treatments that can effectively suppress DKD progression. Finerenone is a first-in-class nonsteroidal mineralocorticoid receptor antagonist with clinically proven efficacy in improving albuminuria, estimated glomerular filtration rate, and risk of cardiovascular events in early and advanced DKD. Therefore, finerenone is a promising treatment option to delay DKD progression. This article reviews the mechanism of renal effects and major clinical outcomes of finerenone in DKD.
Keywords: Diabetes mellitus, type 2; Diabetic nephropathies; Finerenone; Mineralocorticoid receptor antagonists; Renal insufficiency, chronic.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

References
-
- The Korean Society of Nephrology . Seoul: The Korean Society of Nephrology; 2022. Trends in epidemiologic characteristics of end-stage renal disease from 2020 KORDS (Korean Renal Data System) [Internet] [cited 2023 Jan 31]. Available from: https://ksn.or.kr.
-
- Atkins RC. The epidemiology of chronic kidney disease. Kidney Int Suppl. 2005;94:S14–8. - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4S):S1–115. - PubMed
-
- Fried LF, Folkerts K, Smeta B, Bowrin KD, Mernagh P, Millier A, et al. Targeted literature review of the burden of illness in patients with chronic kidney disease and type 2 diabetes. Am J Manag Care. 2021;27(8 Suppl):S168–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

