ETHYLENE-INSENSITIVE 3-LIKE 2 regulates β-carotene and ascorbic acid accumulation in tomatoes during ripening
- PMID: 36891812
- PMCID: PMC10315317
- DOI: 10.1093/plphys/kiad151
ETHYLENE-INSENSITIVE 3-LIKE 2 regulates β-carotene and ascorbic acid accumulation in tomatoes during ripening
Abstract
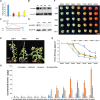
ETHYLENE-INSENSITIVE 3/ETHYLENE-INSENSITIVE 3-LIKEs (EIN3/EILs) are important ethylene response factors during fruit ripening. Here, we discovered that EIL2 controls carotenoid metabolism and ascorbic acid (AsA) biosynthesis in tomato (Solanum lycopersicum). In contrast to the red fruits presented in the wild type (WT) 45 d after pollination, the fruits of CRISPR/Cas9 eil2 mutants and SlEIL2 RNA interference lines (ERIs) showed yellow or orange fruits. Correlation analysis of transcriptome and metabolome data for the ERI and WT ripe fruits revealed that SlEIL2 is involved in β-carotene and AsA accumulation. ETHYLENE RESPONSE FACTORs (ERFs) are the typical components downstream of EIN3 in the ethylene response pathway. Through a comprehensive screening of ERF family members, we determined that SlEIL2 directly regulates the expression of 4 SlERFs. Two of these, SlERF.H30 and SlERF.G6, encode proteins that participate in the regulation of LYCOPENE-β-CYCLASE 2 (SlLCYB2), encoding an enzyme that mediates the conversion of lycopene to carotene in fruits. In addition, SlEIL2 transcriptionally repressed L-GALACTOSE 1-PHOSPHATE PHOSPHATASE 3 (SlGPP3) and MYO-INOSITOL OXYGENASE 1 (SlMIOX1) expression, which resulted in a 1.62-fold increase of AsA via both the L-galactose and myoinositol pathways. Overall, we demonstrated that SlEIL2 functions in controlling β-carotene and AsA levels, providing a potential strategy for genetic engineering to improve the nutritional value and quality of tomato fruit.
© American Society of Plant Biologists 2023. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest statement. The authors declare that there is no conflict of interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

