Insufficiency of Mrpl40 disrupts testicular structure and semen parameters in a murine model
- PMID: 36891938
- PMCID: PMC10521951
- DOI: 10.4103/aja2022119
Insufficiency of Mrpl40 disrupts testicular structure and semen parameters in a murine model
Abstract
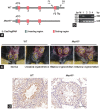
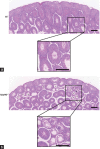
Approximately 31% of patients with 22q11.2 deletion syndrome (22q11.2DS) have genitourinary system disorders and 6% of them have undescended testes. Haploinsufficiency of genes on chromosome 22q11.2 might contribute to the risk of 22q11.2DS. In this study, we used mice with single-allele deletion in mitochondrial ribosomal protein L40 ( Mrpl40 +/- ) as models to investigate the function of Mrpl40 in testes and spermatozoa development. The penetrance of cryptorchidism in Mrpl40 +/- mice was found to be higher than that in wild-type (WT) counterparts. Although the weight of testes was not significantly different between the WT and Mrpl40 +/- mice, the structure of seminiferous tubules and mitochondrial morphology was altered in the Mrpl40 +/- mice. Moreover, the concentration and motility of spermatozoa were significantly decreased in the Mrpl40 +/- mice. In addition, data-independent acquisition mass spectrometry indicated that the expression of genes associated with male infertility was altered in Mrpl40 +/- testes. Our study demonstrated the important role of Mrpl40 in testicular structure and spermatozoa motility and count. These findings suggest that Mrpl40 is potentially a novel therapeutic target for cryptorchidism and decreased motility and count of spermatozoa.
Copyright © 2023 Copyright: © The Author(s)(2023).
Conflict of interest statement
All authors declare no competing interests.
Figures


References
-
- Wu HY, Rusnack SL, Bellah RD, Plachter N, McDonald-McGinn DM, et al. Genitourinary malformations in chromosome 22q11.2 deletion. J Urol. 2002;168:2564–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

