Neutrophil Extracellular Traps in Cerebral Ischemia/Reperfusion Injury: Friend and Foe
- PMID: 36892020
- PMCID: PMC10556361
- DOI: 10.2174/1570159X21666230308090351
Neutrophil Extracellular Traps in Cerebral Ischemia/Reperfusion Injury: Friend and Foe
Abstract
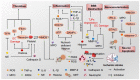
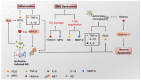
Cerebral ischemic injury, one of the leading causes of morbidity and mortality worldwide, triggers various central nervous system (CNS) diseases, including acute ischemic stroke (AIS) and chronic ischemia-induced Alzheimer's disease (AD). Currently, targeted therapies are urgently needed to address neurological disorders caused by cerebral ischemia/reperfusion injury (CI/RI), and the emergence of neutrophil extracellular traps (NETs) may be able to relieve the pressure. Neutrophils are precursors to brain injury following ischemic stroke and exert complicated functions. NETs extracellularly release reticular complexes of neutrophils, i.e., double-stranded DNA (dsDNA), histones, and granulins. Paradoxically, NETs play a dual role, friend and foe, under different conditions, for example, physiological circumstances, infection, neurodegeneration, and ischemia/reperfusion. Increasing evidence indicates that NETs exert anti-inflammatory effects by degrading cytokines and chemokines through protease at a relatively stable and moderate level under physiological conditions, while excessive amounts of NETs release (NETosis) irritated by CI/RI exacerbate the inflammatory response and aggravate thrombosis, disrupt the blood-brain barrier (BBB), and initiates sequential neuron injury and tissue damage. This review provides a comprehensive overview of the machinery of NETs formation and the role of an abnormal cascade of NETs in CI/RI, as well as other ischemia-induced neurological diseases. Herein, we highlight the potential of NETs as a therapeutic target against ischemic stroke that may inspire translational research and innovative clinical approaches.
Keywords: Alzheimer’s disease; CNS diseases; Ischemic stroke; cerebral ischemia; cerebral ischemia-reperfusion; neutrophil extracellular traps.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures
Similar articles
-
Neutrophil Extracellular Traps Exacerbate Ischemic Brain Damage.Mol Neurobiol. 2022 Jan;59(1):643-656. doi: 10.1007/s12035-021-02635-z. Epub 2021 Nov 8. Mol Neurobiol. 2022. PMID: 34748205 Review.
-
Neutrophil extracellular traps and citrullinated fibrinogen contribute to injury in a porcine model of limb ischemia and reperfusion.Front Immunol. 2024 Sep 9;15:1436926. doi: 10.3389/fimmu.2024.1436926. eCollection 2024. Front Immunol. 2024. PMID: 39315100 Free PMC article.
-
The Brain Entangled: The Contribution of Neutrophil Extracellular Traps to the Diseases of the Central Nervous System.Cells. 2019 Nov 21;8(12):1477. doi: 10.3390/cells8121477. Cells. 2019. PMID: 31766346 Free PMC article. Review.
-
Neutrophil extracellular traps contribute to tissue plasminogen activator resistance in acute ischemic stroke.FASEB J. 2021 Sep;35(9):e21835. doi: 10.1096/fj.202100471RR. FASEB J. 2021. PMID: 34449927
-
[Effect of neutrophil extracellular traps on ischemic stroke and intervention of traditional Chinese medicine].Zhongguo Zhong Yao Za Zhi. 2021 Jan;46(1):1-5. doi: 10.19540/j.cnki.cjcmm.20200908.601. Zhongguo Zhong Yao Za Zhi. 2021. PMID: 33645044 Review. Chinese.
Cited by
-
Research progress on remote ischemic conditioning for improving prognosis of patients with acute ischemic stroke.Biochem Biophys Rep. 2025 Jul 30;43:102184. doi: 10.1016/j.bbrep.2025.102184. eCollection 2025 Sep. Biochem Biophys Rep. 2025. PMID: 40791818 Free PMC article. Review.
-
Targeting revascularization via tryptophan-indole-NETs axis: the synergistic power of acupuncture and rt-PA thrombolysis in ischemic stroke.Front Neurol. 2025 Jul 4;16:1596158. doi: 10.3389/fneur.2025.1596158. eCollection 2025. Front Neurol. 2025. PMID: 40689325 Free PMC article.
-
Acteoside ameliorates cerebral ischemia/reperfusion injury in MCAO rats as a natural plasma Kallikrein inhibitor from Osmanthus fragrans flower.Sci Rep. 2025 Jul 2;15(1):23509. doi: 10.1038/s41598-025-06553-1. Sci Rep. 2025. PMID: 40603384 Free PMC article.
-
Bioinformatics insights into mitochondrial and immune gene regulation in Alzheimer's disease.Eur J Med Res. 2025 Feb 8;30(1):89. doi: 10.1186/s40001-025-02297-w. Eur J Med Res. 2025. PMID: 39920860 Free PMC article.
-
Neutrophil extracellular traps in homeostasis and disease.Signal Transduct Target Ther. 2024 Sep 20;9(1):235. doi: 10.1038/s41392-024-01933-x. Signal Transduct Target Ther. 2024. PMID: 39300084 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

