Narrative Review of Drug-Associated Nail Toxicities in Oncologic Patients
- PMID: 36892360
- PMCID: PMC9946059
- DOI: 10.5826/dpc.1301a64
Narrative Review of Drug-Associated Nail Toxicities in Oncologic Patients
Abstract
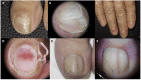
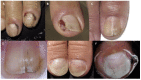
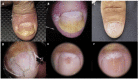
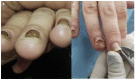
Introduction: Nail toxicity represents one of the most common cutaneous adverse effects of both classic chemotherapeutic agents and new oncologic drugs, including targeted treatments and immunotherapy.
Objectives: We aimed to provide a comprehensive literature review of nail toxicities derived from conventional chemotherapeutic agents, targeted therapies (EGFR inhibitors, multikinase inhibitors, BRAF and MEK inhibitors) and immune checkpoint inhibitors (ICIs), including clinical presentation, implicated drugs and approaches for prevention and management.
Methods: Retrieved literature from PubMed registry database was reviewed to include all articles published up to May 2021 relevant to the clinical presentation, diagnosis, incidence, prevention, and treatment of oncologic treatment-induced nail toxicity. The internet was searched for relevant studies.
Results: A wide spectrum of nail toxicities is associated with both, conventional and newer anticancer agents. The frequency of nail involvement, especially with immunotherapy and new targeted agents remains unknown and patients with different cancer types receiving different regimens may develop the same nail disorder, whereas patients with the same type of cancer under the same chemotherapeutic treatment may develop different types of nail alterations. The underlying mechanisms of the varying individual susceptibility and the diverse nail responses to various anticancer treatments need further investigation.
Conclusion: Early recognition and treatment of nail toxicities can minimize their impact, allowing better adherence to conventional and newer oncologic treatments. Dermatologists, oncologists and other implicated physicians should be aware of these burdensome adverse effects in order to guide management and prevent impairment of patients' quality of life.
Conflict of interest statement
Figures
References
-
- Pavey R, Kambil S, Bhat R. Dermatological adverse reactions to cancer chemotherapy. Indian J Dermatol Venereol Leprol. 2015;81(4):434. - PubMed
-
- Piraccini BM, Tosti A. Drug-induced nail disorders. Incidence, management and prognosis. Drug Saf. 1999;21(3):187–201. - PubMed
-
- Hinds G, Thomas VD. Malignancy and Cancer Treatment-Related Hair and Nail Changes. Dermatol Clin. 2008;26(1):59–68. - PubMed
-
- Saraswat N, Sood A, Verma R, Kumar D, Kumar S. Nail changes induced by chemotherapeutic agents. Indian J Dermatol [Internet] 2020 May 1;65(3):193. [cited 2021 Apr 18] Available from: http://www.e-ijd.org/text.asp?2020/65/3/193/282451. - PMC - PubMed
-
- Daniel CR, Scher RK. Nail changes secondary to systemic drugs or ingestants. J Am Acad Dermatol. 1984;10(2):250–8. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
