Development of a Common Data Model for a Multisite and Multiyear Study of Virtual Visit Implementation: A Case Study
- PMID: 36893419
- PMCID: PMC9994571
- DOI: 10.1097/MLR.0000000000001834
Development of a Common Data Model for a Multisite and Multiyear Study of Virtual Visit Implementation: A Case Study
Abstract
Background/objective: In multisite studies, a common data model (CDM) standardizes dataset organization, variable definitions, and variable code structures and can support distributed data processing. We describe the development of a CDM for a study of virtual visit implementation in 3 Kaiser Permanente (KP) regions.
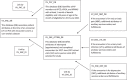
Methods: We conducted several scoping reviews to inform our study's CDM design: (1) virtual visit mode, implementation timing, and scope (targeted clinical conditions and departments); and (2) extant sources of electronic health record data to specify study measures. Our study covered the period from 2017 through June 2021. Integrity of the CDM was assessed by a chart review of random samples of virtual and in-person visits, overall and by specific conditions of interest (neck or back pain, urinary tract infection, major depression).
Results: The scoping reviews identified a need to address differences in virtual visit programs across the 3 KP regionsto harmonize measurement specifications for our research analyses. The final CDM contained patient-level, provider-level, and system-level measures on 7,476,604 person-years for KP members aged 19 years and above. Utilization included 2,966,112 virtual visits (synchronous chats, telephone visits, video visits) and 10,004,195 in-person visits. Chart review indicated the CDM correctly identified visit mode on>96% (n=444) of visits, and presenting diagnosis on >91% (n=482) of visits.
Conclusions: Upfront design and implementation of CDMs may be resource intensive. Once implemented, CDMs, like the one we developed for our study, provide downstream programming and analytic efficiencies by harmonizing, in a consistent framework, otherwise idiosyncratic temporal and study site differences in source data.
Copyright © 2023 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Johnson III RJ. A comprehensive review of an electronic health record soon to assume market ascendency: EPIC. J Health Commun. 2016;1:36.
-
- HealthIT.gov. Epic Interoperability Fact Sheet. 2014. Accessed December 15, 2019. https://www.healthit.gov/sites/default/files/facas/GSG_TestimonySupport_....
-
- Mattison JE, Dolin RH, Laberge D. Managing the tensions between national standardization vs. regional localization of clinical content and templates. Stud Health Technol Inform. 2004;107(pt 2):1081–1085. - PubMed
-
- Raymond B. The Kaiser Permanente IT transformation. Healthc Financ Manage. 2005;59:62–66. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous