Candida albicans-specific Th17 cell-mediated response contributes to alcohol-associated liver disease
- PMID: 36893735
- PMCID: PMC10039706
- DOI: 10.1016/j.chom.2023.02.001
Candida albicans-specific Th17 cell-mediated response contributes to alcohol-associated liver disease
Abstract
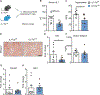
Alcohol-associated liver disease is accompanied by intestinal mycobiome dysbiosis, yet the impacts on liver disease are unclear. We demonstrate that Candida albicans-specific T helper 17 (Th17) cells are increased in circulation and present in the liver of patients with alcohol-associated liver disease. Chronic ethanol administration in mice causes migration of Candida albicans (C. albicans)-reactive Th17 cells from the intestine to the liver. The antifungal agent nystatin decreased C. albicans-specific Th17 cells in the liver and reduced ethanol-induced liver disease in mice. Transgenic mice expressing T cell receptors (TCRs) reactive to Candida antigens developed more severe ethanol-induced liver disease than transgene-negative littermates. Adoptively transferring Candida-specific TCR transgenic T cells or polyclonal C. albicans-primed T cells exacerbated ethanol-induced liver disease in wild-type mice. Interleukin-17 (IL-17) receptor A signaling in Kupffer cells was required for the effects of polyclonal C. albicans-primed T cells. Our findings indicate that ethanol increases C. albicans-specific Th17 cells, which contribute to alcohol-associated liver disease.
Keywords: ALD; alcoholic liver disease; microbiome; microbiota; mycobiome.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests B.S. has been consulting for Ambys Medicines, Ferring Research Institute, Gelesis, HOST Therabiomics, Intercept Pharmaceuticals, Mabwell Therapeutics, Patara Pharmaceuticals, and Takeda. B.S.’s institution UC San Diego has received research support from Artizan Biosciences, Axial Biotherapeutics, BiomX, CymaBay Therapeutics, NGM Biopharmaceuticals, Prodigy Biotech, and Synlogic Operating Company. B.S. is the founder of Nterica Bio. UC San Diego has filed several patents with B.S. and Y.D. as inventors related to this work. P.S. received grant support from Gilead Sciences Belgium.
Figures






References
MeSH terms
Substances
Grants and funding
- R35 HL139930/HL/NHLBI NIH HHS/United States
- R01 HL141999/HL/NHLBI NIH HHS/United States
- I01 BX004594/BX/BLRD VA/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- P30 DK120515/DK/NIDDK NIH HHS/United States
- R01 DK099205/DK/NIDDK NIH HHS/United States
- R01 AA028550/AA/NIAAA NIH HHS/United States
- R01 GM124494/GM/NIGMS NIH HHS/United States
- P50 AA011999/AA/NIAAA NIH HHS/United States
- U01 AA026939/AA/NIAAA NIH HHS/United States
- U01 AA029019/AA/NIAAA NIH HHS/United States
- R01 AA024726/AA/NIAAA NIH HHS/United States
- R01 DK091183/DK/NIDDK NIH HHS/United States
- R37 AA020703/AA/NIAAA NIH HHS/United States
- KL2 TR001444/TR/NCATS NIH HHS/United States
- R01 DK101737/DK/NIDDK NIH HHS/United States
- R44 DK115242/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

