Choroid plexus-targeted NKCC1 overexpression to treat post-hemorrhagic hydrocephalus
- PMID: 36893755
- PMCID: PMC10198810
- DOI: 10.1016/j.neuron.2023.02.020
Choroid plexus-targeted NKCC1 overexpression to treat post-hemorrhagic hydrocephalus
Abstract
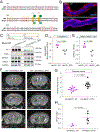
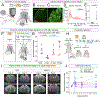
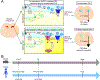
Post-hemorrhagic hydrocephalus (PHH) refers to a life-threatening accumulation of cerebrospinal fluid (CSF) that occurs following intraventricular hemorrhage (IVH). An incomplete understanding of this variably progressive condition has hampered the development of new therapies beyond serial neurosurgical interventions. Here, we show a key role for the bidirectional Na-K-Cl cotransporter, NKCC1, in the choroid plexus (ChP) to mitigate PHH. Mimicking IVH with intraventricular blood led to increased CSF [K+] and triggered cytosolic calcium activity in ChP epithelial cells, which was followed by NKCC1 activation. ChP-targeted adeno-associated viral (AAV)-NKCC1 prevented blood-induced ventriculomegaly and led to persistently increased CSF clearance capacity. These data demonstrate that intraventricular blood triggered a trans-choroidal, NKCC1-dependent CSF clearance mechanism. Inactive, phosphodeficient AAV-NKCC1-NT51 failed to mitigate ventriculomegaly. Excessive CSF [K+] fluctuations correlated with permanent shunting outcome in humans following hemorrhagic stroke, suggesting targeted gene therapy as a potential treatment to mitigate intracranial fluid accumulation following hemorrhage.
Keywords: Cerebrospinal fluid; NKCC1; adeno-associated virus; choroid plexus; epithelial cells; gene therapy; hydrocephalus; intraventricular hemorrhage; ventricles.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.K.L., R.M.F., C.S., and H.X. are co-inventors on a provisional patent application related to this manuscript.
Figures




Comment in
-
Boosting NKCC1 in the choroid plexus: From CSF clearance to a potential therapy for posthemorrhagic hydrocephalus.Neuron. 2023 May 17;111(10):1521-1523. doi: 10.1016/j.neuron.2023.04.014. Neuron. 2023. PMID: 37201502
References
-
- Kulkarni AV, Riva-Cambrin J, Butler J, Browd SR, Drake JM, Holubkov R, Kestle JR, Limbrick DD, Simon TD, Tamber MS, et al. (2013). Outcomes of CSF shunting in children: comparison of Hydrocephalus Clinical Research Network cohort with historical controls: clinical article. J Neurosurg Pediatr 12, 334–338. 10.3171/2013.7.PEDS12637. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS088566/NS/NINDS NIH HHS/United States
- R01 NS129823/NS/NINDS NIH HHS/United States
- P50 HD105351/HD/NICHD NIH HHS/United States
- T32 HL007901/HL/NHLBI NIH HHS/United States
- DP1 AT010971/AT/NCCIH NIH HHS/United States
- P30 EY012196/EY/NEI NIH HHS/United States
- F31 NS115369/NS/NINDS NIH HHS/United States
- T32 GM144273/GM/NIGMS NIH HHS/United States
- T32 GM008313/GM/NIGMS NIH HHS/United States
- R35 HL145242/HL/NHLBI NIH HHS/United States
- U24 AI152179/AI/NIAID NIH HHS/United States
- R01 AI130591/AI/NIAID NIH HHS/United States
- R01 HD096693/HD/NICHD NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- T32 HL110852/HL/NHLBI NIH HHS/United States
- F30 DK131642/DK/NIDDK NIH HHS/United States
- U54 HD090255/HD/NICHD NIH HHS/United States
- RF1 DA048790/DA/NIDA NIH HHS/United States
- R00 HD083512/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

