Oxygen toxicity causes cyclic damage by destabilizing specific Fe-S cluster-containing protein complexes
- PMID: 36893757
- PMCID: PMC10148707
- DOI: 10.1016/j.molcel.2023.02.013
Oxygen toxicity causes cyclic damage by destabilizing specific Fe-S cluster-containing protein complexes
Abstract
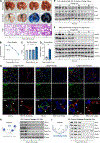
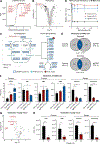
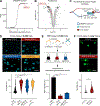
Oxygen is toxic across all three domains of life. Yet, the underlying molecular mechanisms remain largely unknown. Here, we systematically investigate the major cellular pathways affected by excess molecular oxygen. We find that hyperoxia destabilizes a specific subset of Fe-S cluster (ISC)-containing proteins, resulting in impaired diphthamide synthesis, purine metabolism, nucleotide excision repair, and electron transport chain (ETC) function. Our findings translate to primary human lung cells and a mouse model of pulmonary oxygen toxicity. We demonstrate that the ETC is the most vulnerable to damage, resulting in decreased mitochondrial oxygen consumption. This leads to further tissue hyperoxia and cyclic damage of the additional ISC-containing pathways. In support of this model, primary ETC dysfunction in the Ndufs4 KO mouse model causes lung tissue hyperoxia and dramatically increases sensitivity to hyperoxia-mediated ISC damage. This work has important implications for hyperoxia pathologies, including bronchopulmonary dysplasia, ischemia-reperfusion injury, aging, and mitochondrial disorders.
Keywords: DNA damage; Fe-S clusters; hyperoxia; lung injury; mitochondria; oxygen; purine synthesis; redox; translation fidelity.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests I.H.J. is a consultant for Maze Therapeutics and has patents related to hypoxia therapy for metabolic disorders.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

