Ferroptosis Suppressor Protein 1 Inhibition Promotes Tumor Ferroptosis and Anti-tumor Immune Responses in Liver Cancer
- PMID: 36893885
- PMCID: PMC10230009
- DOI: 10.1016/j.jcmgh.2023.03.001
Ferroptosis Suppressor Protein 1 Inhibition Promotes Tumor Ferroptosis and Anti-tumor Immune Responses in Liver Cancer
Abstract
Background & aims: Hepatocellular carcinoma (HCC) is a highly aggressive malignancy with dreadful clinical outcome. Tyrosine kinase inhibitors and immune checkpoint inhibitors are the only United States Food and Drug Administration-approved therapeutic options for patients with advanced HCC with limited therapeutic success. Ferroptosis is a form of immunogenic and regulated cell death caused by chain reaction of iron-dependent lipid peroxidation. Coenzyme Q10 (CoQ10)/ferroptosis suppressor protein 1 (FSP1) axis was recently identified as a novel protective mechanism against ferroptosis. We would like to explore whether FSP1 could be a potential therapeutic target for HCC.
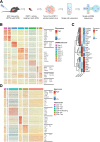
Methods: FSP1 expression in human HCC and paired non-tumorous tissue samples were determined by reverse transcription-quantitative polymerase chain reaction, followed by clinicopathologic correlation and survival studies. Regulatory mechanism for FSP1 was determined using chromatin immunoprecipitation. The hydrodynamic tail vein injection model was used for HCC induction to evaluate the efficacy of FSP1 inhibitor (iFSP1) in vivo. Single-cell RNA sequencing revealed the immunomodulatory effects of iFSP1 treatment.
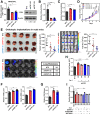
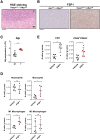
Results: We showed that HCC cells greatly rely on the CoQ10/FSP1 system to overcome ferroptosis. We found that FSP1 was significantly overexpressed in human HCC and is regulated by kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 pathway. FSP1 inhibitor iFSP1 effectively reduced HCC burden and profoundly increased immune infiltrates including dendritic cells, macrophages, and T cells. We also demonstrated that iFSP1 worked synergistically with immunotherapies to suppress HCC progression.
Conclusions: We identified FSP1 as a novel, vulnerable therapeutic target in HCC. The inhibition of FSP1 potently induced ferroptosis, which promoted innate and adaptive anti-tumor immune responses and effectively suppressed HCC tumor growth. FSP1 inhibition therefore represents a new therapeutic strategy for HCC.
Keywords: Hepatocellular Carcinoma; Immunogenic Cell Death; Lipid Peroxidation; Regulated Cell Death.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures










References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Llovet J.M., Kelley R.K., Villanueva A., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

