Initiation and modulation of Tau protein phase separation by the drug suramin
- PMID: 36894559
- PMCID: PMC9997437
- DOI: 10.1038/s41598-023-29846-9
Initiation and modulation of Tau protein phase separation by the drug suramin
Abstract
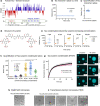
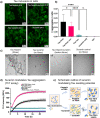
Tau is an intrinsically disordered neuronal protein in the central nervous system. Aggregated Tau is the main component of neurofibrillary tangles observed in Alzheimer's disease. In vitro, Tau aggregation can be triggered by polyanionic co-factors, like RNA or heparin. At different concentration ratios, the same polyanions can induce Tau condensates via liquid-liquid phase separation (LLPS), which over time develop pathological aggregation seeding potential. Data obtained by time resolved Dynamic Light Scattering experiments (trDLS), light and electron microscopy show that intermolecular electrostatic interactions between Tau and the negatively charged drug suramin induce Tau condensation and compete with the interactions driving and stabilizing the formation of Tau:heparin and Tau:RNA coacervates, thus, reducing their potential to induce cellular Tau aggregation. Tau:suramin condensates do not seed Tau aggregation in a HEK cell model for Tau aggregation, even after extended incubation. These observations indicate that electrostatically driven Tau condensation can occur without pathological aggregation when initiated by small anionic molecules. Our results provide a novel avenue for therapeutic intervention of aberrant Tau phase separation, utilizing small anionic compounds.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

