Sustained overexpression of spliced X-box-binding protein-1 in neurons leads to spontaneous seizures and sudden death in mice
- PMID: 36894627
- PMCID: PMC9998612
- DOI: 10.1038/s42003-023-04594-8
Sustained overexpression of spliced X-box-binding protein-1 in neurons leads to spontaneous seizures and sudden death in mice
Abstract
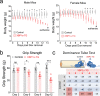
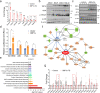
The underlying etiologies of seizures are highly heterogeneous and remain incompletely understood. While studying the unfolded protein response (UPR) pathways in the brain, we unexpectedly discovered that transgenic mice (XBP1s-TG) expressing spliced X-box-binding protein-1 (Xbp1s), a key effector of UPR signaling, in forebrain excitatory neurons, rapidly develop neurologic deficits, most notably recurrent spontaneous seizures. This seizure phenotype begins around 8 days after Xbp1s transgene expression is induced in XBP1s-TG mice, and by approximately 14 days post induction, the seizures evolve into status epilepticus with nearly continuous seizure activity followed by sudden death. Animal death is likely due to severe seizures because the anticonvulsant valproic acid could significantly prolong the lives of XBP1s-TG mice. Mechanistically, our gene profiling analysis indicates that compared to control mice, XBP1s-TG mice exhibit 591 differentially regulated genes (mostly upregulated) in the brain, including several GABAA receptor genes that are notably downregulated. Finally, whole-cell patch clamp analysis reveals a significant reduction in both spontaneous and tonic GABAergic inhibitory responses in Xbp1s-expressing neurons. Taken together, our findings unravel a link between XBP1s signaling and seizure occurrence.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

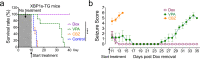


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

