Benchmarking of survival outcomes following Haematopoietic Stem Cell Transplantation (HSCT): an update of the ongoing project of the European Society for Blood and Marrow Transplantation (EBMT) and Joint Accreditation Committee of ISCT and EBMT (JACIE)
- PMID: 36894635
- PMCID: PMC9995719
- DOI: 10.1038/s41409-023-01924-6
Benchmarking of survival outcomes following Haematopoietic Stem Cell Transplantation (HSCT): an update of the ongoing project of the European Society for Blood and Marrow Transplantation (EBMT) and Joint Accreditation Committee of ISCT and EBMT (JACIE)
Abstract
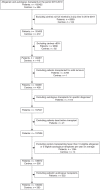
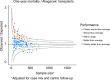
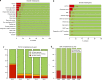
From 2016 EBMT and JACIE developed an international risk-adapted benchmarking program of haematopoietic stem cell transplant (HSCT) outcome to provide individual EBMT Centers with a means of quality-assuring the HSCT process and meeting FACT-JACIE accreditation requirements relating to 1-year survival outcomes. Informed by previous experience from Europe, North America and Australasia, the Clinical Outcomes Group (COG) established criteria for patient and Center selection, and a set of key clinical variables within a dedicated statistical model adapted to the capabilities of the EBMT Registry. The first phase of the project was launched in 2019 to test the acceptability of the benchmarking model through assessment of Centers' performance for 1-year data completeness and survival outcomes of autologous and allogeneic HSCT covering 2013-2016. A second phase was delivered in July 2021 covering 2015-2019 and including survival outcomes. Reports of individual Center performance were shared directly with local principal investigators and their responses were assimilated. The experience thus far has supported the feasibility, acceptability and reliability of the system as well as identifying its limitations. We provide a summary of experience and learning so far in this 'work in progress', as well as highlighting future challenges of delivering a modern, robust, data-complete, risk-adapted benchmarking program across new EBMT Registry systems.
© 2023. The Author(s).
Conflict of interest statement
AMSB declares honoraria from Takeda, BMS/Celgene, MSD, Janssen, Amgen, Novartis, Gilead Kite, Sanofi, Roche, Alexion and Consultancy: Takeda, BMS/Celgene, Novartis, Janssen, Gilead, Sanofi, Speaker’s Bureau: Takeda and Research Support from Takeda, BMS/Celgene. GA declares paid consultancy project for Pfizer in 2021. MK declares honoraria for educational events from Jazz and Mallinkrodt, for advisory board membership from Jazz. RPdL declares Expert consultant/speaker: Alexion, Amgen, Gilead, Jazz, Keocyte, MSD, Novartis, Pfizer, Roche, Samsung & Therakos. Research grant: Alexion, Amgen, Jazz pharmaceutical, Novartis, Pfizer. Rémy Dulery declares personal fees from Takeda, Novartis, Incyte and Biotest and non-financial support from Gilead, (not related the submitted work). S-G, Fermindeclares honoraria and/or research support from Novartis, Kite/Gilead, Celgene/BMS, Pfizer, Incyte, Amgen, Takeda and Roche. RS declares honoraria and/or research support from Novartis, Kite/Gilead, Jazz Pharmaceuticals, Mallinkrodt, Sanofi and Cytiva. JS declares honoraria for educational events from Gilead and Janssen, for advisory board membership from Medac, and for trial IDMC membership from Kiadis Pharma.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources

