A novel cuproptosis-related gene model predicts outcomes and treatment responses in pancreatic adenocarcinoma
- PMID: 36894917
- PMCID: PMC9999523
- DOI: 10.1186/s12885-023-10678-9
A novel cuproptosis-related gene model predicts outcomes and treatment responses in pancreatic adenocarcinoma
Abstract
Background: Cuproptosis is recently emerging as a hot spot in cancer research. However, its role in pancreatic adenocarcinoma (PAAD) has not yet been clarified. This study aimed to explore the prognostic and therapeutic implications of cuproptosis-related genes in PAAD.
Methods: Two hundred thirteen PAAD samples from the International Cancer Genome Consortium (ICGC) were split into training and validation sets in the ratio of 7:3. The Cox regression analyses generated a prognostic model using the ICGC cohort for training (n = 152) and validation (n = 61). The model was externally tested on the Gene Expression Omnibus (GEO) (n = 80) and The Cancer Genome Atlas (TCGA) datasets (n = 176). The clinical characteristics, molecular mechanisms, immune landscape, and treatment responses in model-defined subgroups were explored. The expression of an independent prognostic gene TSC22D2 was confirmed by public databases, real-time quantitative PCR (RT-qPCR), western blot (WB), and immunohistochemistry (IHC).
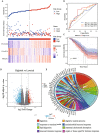
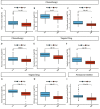
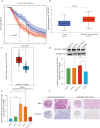
Results: A prognostic model was established based on three cuproptosis-related genes (TSC22D2, C6orf136, PRKDC). Patients were stratified into high- and low-risk groups using the risk score based on this model. PAAD patients in the high-risk group had a worse prognosis. The risk score was statistically significantly correlated with most clinicopathological characteristics. The risk score based on this model was an independent predictor of overall survival (OS) (HR = 10.7, p < 0.001), and was utilized to create a scoring nomogram with excellent prognostic value. High-risk patients had a higher TP53 mutation rate and a superior response to multiple targeted therapies and chemotherapeutic drugs, but might obtain fewer benefits from immunotherapy. Moreover, elevated TSC22D2 expression was discovered to be an independent prognostic predictor for OS (p < 0.001). Data from public databases and our own experiments showed that TSC22D2 expression was significantly higher in pancreatic cancer tissues/cells compared to normal tissues/cells.
Conclusion: This novel model based on cuproptosis-related genes provided a robust biomarker for predicting the prognosis and treatment responses of PAAD. The potential roles and underlying mechanisms of TSC22D2 in PAAD need further explored.
Keywords: Cuproptosis; Immunotherapy; Pancreatic adenocarcinoma; Prognosis; Risk model; Treatment response; Tumor microenvironment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

