Diagnostic accuracy of a set of clinical and radiological criteria for screening of COVID-19 using RT-PCR as the reference standard
- PMID: 36894945
- PMCID: PMC9997428
- DOI: 10.1186/s12890-023-02369-9
Diagnostic accuracy of a set of clinical and radiological criteria for screening of COVID-19 using RT-PCR as the reference standard
Abstract
Background: The gold-standard method for establishing a microbiological diagnosis of COVID-19 is reverse-transcriptase polymerase chain reaction (RT-PCR). This study aimed to evaluate the accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of a set of clinical-radiological criteria for COVID-19 screening in patients with severe acute respiratory failure (SARF) admitted to intensive care units (ICUs), using reverse-transcriptase polymerase chain reaction (RT-PCR) as the reference standard.
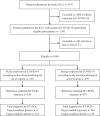
Methods: Diagnostic accuracy study including a historical cohort of 1009 patients consecutively admitted to ICUs across six hospitals in Curitiba (Brazil) from March to September, 2020. The sample was stratified into groups by the strength of suspicion for COVID-19 (strong versus weak) using parameters based on three clinical and radiological (chest computed tomography) criteria. The diagnosis of COVID-19 was confirmed by RT-PCR (referent).
Results: With respect to RT-PCR, the proposed criteria had 98.5% (95% confidence interval [95% CI] 97.5-99.5%) sensitivity, 70% (95% CI 65.8-74.2%) specificity, 85.5% (95% CI 83.4-87.7%) accuracy, PPV of 79.7% (95% CI 76.6-82.7%) and NPV of 97.6% (95% CI 95.9-99.2%). Similar performance was observed when evaluated in the subgroups of patients admitted with mild/moderate respiratory disfunction, and severe respiratory disfunction.
Conclusion: The proposed set of clinical-radiological criteria were accurate in identifying patients with strong versus weak suspicion for COVID-19 and had high sensitivity and considerable specificity with respect to RT-PCR. These criteria may be useful for screening COVID-19 in patients presenting with SARF.
Keywords: COVID-19 testing; Intensive care units; Mass screening; Sensitivity and specificity; Tomography.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- China National Health Commission. Diagnosis and treatment protocol for COVID-19 (Trial Version 7) [Internet]. Beijing: China National Health Commission. 2020 [cited 2022 Jun 15]. Available from: http://en.nhc.gov.cn/2020-03/29/c_78469.htm
MeSH terms
LinkOut - more resources
Full Text Sources
Medical