Tunable Mesoscopic Collagen Island Architectures Modulate Stem Cell Behavior
- PMID: 36895051
- PMCID: PMC10166061
- DOI: 10.1002/adma.202207882
Tunable Mesoscopic Collagen Island Architectures Modulate Stem Cell Behavior
Abstract
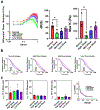
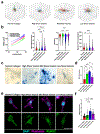
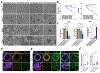
The extracellular matrix is the biophysical environment that scaffolds mammalian cells in the body. The main constituent is collagen. In physiological tissues, collagen network topology is diverse with complex mesoscopic features. While studies have explored the roles of collagen density and stiffness, the impact of complex architectures remains not well-understood. Developing in vitro systems that recapitulate these diverse collagen architectures is critical for understanding physiologically relevant cell behaviors. Here, methods are developed to induce the formation of heterogeneous mesoscopic architectures, referred to as collagen islands, in collagen hydrogels. These island-containing gels have highly tunable inclusions and mechanical properties. Although these gels are globally soft, there is regional enrichment in the collagen concentration at the cell-scale. Collagen-island architectures are utilized to study mesenchymal stem cell behavior, and it is demonstrated that cell migration and osteogenic differentiation are altered. Finally, induced pluripotent stem cells are cultured in island-containing gels, and it is shown that the architecture is sufficient to induce mesodermal differentiation. Overall, this work highlights complex mesoscopic tissue architectures as bioactive cues in regulating cell behavior and presents a novel collagen-based hydrogel that captures these features for tissue engineering applications.
Keywords: biomaterials; cell-extracellular matrix interactions; collagen; tissue engineering.
© 2023 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interest Statement
The authors declare no potential conflicts of interests.
Figures


References
-
- Boraschi-Diaz I, Wang J, Mort JS & Komarova SV Collagen Type I as a Ligand for Receptor-Mediated Signaling. Frontiers in Physics vol. 5 Preprint at 10.3389/fphy.2017.00012 (2017). - DOI
-
- Dupont S et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011). - PubMed
-
- Paszek MJ et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005). - PubMed
-
- Motte S & Kaufman LJ Strain stiffening in collagen I networks. Biopolymers 99, 35–46 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

