Onion plants (Allium cepa L.) react differently to salinity levels according to the regulation of aquaporins
- PMID: 36895341
- PMCID: PMC9988491
- DOI: 10.1016/j.heliyon.2023.e13815
Onion plants (Allium cepa L.) react differently to salinity levels according to the regulation of aquaporins
Abstract
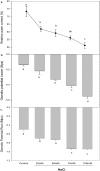
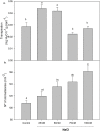
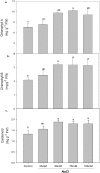
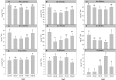
As salinity is one of the main environmental stresses that reduces the growth and productivity of crops by reducing water uptake and transport, in this work, we associated the physiological tolerance response of onion to increased NaCl concentration (from 25, 50, 75, to 100 mM) with the expression of aquaporins. Measurements of transpiration, gas exchange and nutrients content in leaf, roots and bulb tissues were determined in relation to the expression of PIP2, PIP1, and TIP2 aquaporin genes. The results indicated a significant decrease in growth in leaves, roots and bulbs only when 50 mM NaCl was applied. However, this was not correlated with the rest of the parameters, such as transpiration, number of stomata, osmotic potential, or chlorophyll concentration. In this way, the finding that the decreases in Mn, Zn and B observed in leaves, roots and bulbs at 50 mM NaCl were related to the expression of aquaporins, leaded to propose two phases of responses to salinity depending on level of NaCl. Therefore, the activation of PIP2 at 75 mM, in relation to Zn uptake, is proposed as relevant in the response of onion to high salinity.
Keywords: Allium cepa L.; PIP1; PIP2; Salinity; TIP2.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Ashraf M., Harris P. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166:3–16. doi: 10.1016/j.plantsci.2003.10.024. - DOI
-
- Carvajal M., Cooke D., Clarkson D. Water transport across plant plasma membrane. Plant Growth Regul. 1998;25:89–95.
LinkOut - more resources
Full Text Sources
Research Materials

