Strategies to access the [5-8] bicyclic core encountered in the sesquiterpene, diterpene and sesterterpene series
- PMID: 36895430
- PMCID: PMC9989678
- DOI: 10.3762/bjoc.19.23
Strategies to access the [5-8] bicyclic core encountered in the sesquiterpene, diterpene and sesterterpene series
Abstract
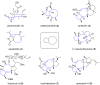
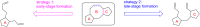
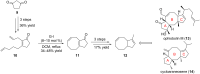
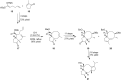
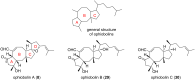
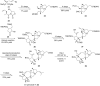
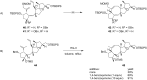
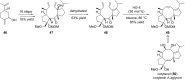
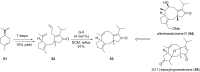
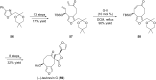
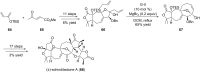
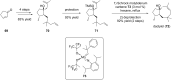
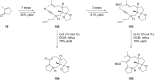
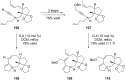
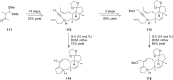
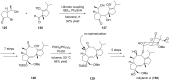
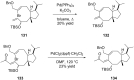
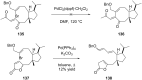
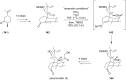
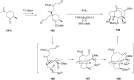
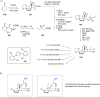
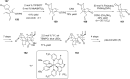
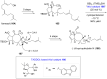
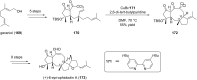
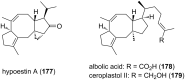
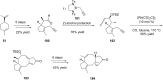
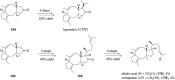
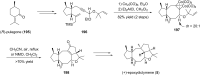
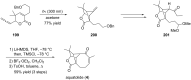
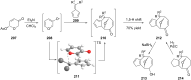
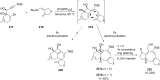
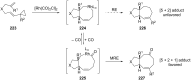
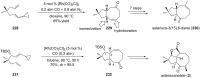
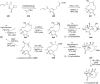
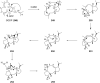
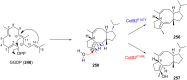
Terpene compounds probably represent the most diversified class of secondary metabolites. Some classes of terpenes, mainly diterpenes (C20) and sesterterpenes (C25) and to a lesser extent sesquiterpenes (C15), share a common bicyclo[3.6.0]undecane core which is characterized by the presence of a cyclooctane ring fused to a cyclopentane ring, i.e., a [5-8] bicyclic ring system. This review focuses on the different strategies elaborated to construct this [5-8] bicyclic ring system and their application in the total synthesis of terpenes over the last two decades. The overall approaches involve the construction of the 8-membered ring from an appropriate cyclopentane precursor. The proposed strategies include metathesis, Nozaki-Hiyama-Kishi (NHK) cyclization, Pd-mediated cyclization, radical cyclization, Pauson-Khand reaction, Lewis acid-promoted cyclization, rearrangement, cycloaddition and biocatalysis.
Keywords: 5-8 bicycle; cyclization strategies; terpenes.
Copyright © 2023, Alleman et al.
Figures
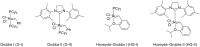

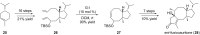
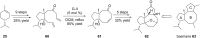



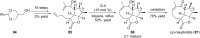
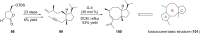

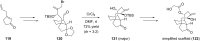



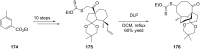

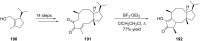
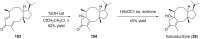
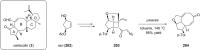
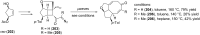
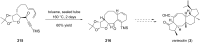

Similar articles
-
Syntheses of Complex Terpenes from Simple Polyprenyl Precursors.Acc Chem Res. 2020 Apr 21;53(4):949-961. doi: 10.1021/acs.accounts.0c00055. Epub 2020 Mar 23. Acc Chem Res. 2020. PMID: 32202757 Free PMC article. Review.
-
Diverged Plant Terpene Synthases Reroute the Carbocation Cyclization Path towards the Formation of Unprecedented 6/11/5 and 6/6/7/5 Sesterterpene Scaffolds.Angew Chem Int Ed Engl. 2018 Jan 26;57(5):1291-1295. doi: 10.1002/anie.201711444. Epub 2017 Dec 28. Angew Chem Int Ed Engl. 2018. PMID: 29194888 Free PMC article.
-
Occurrence and biosynthesis of plant sesterterpenes (C25), a new addition to terpene diversity.Plant Commun. 2021 Mar 30;2(5):100184. doi: 10.1016/j.xplc.2021.100184. eCollection 2021 Sep 13. Plant Commun. 2021. PMID: 34746758 Free PMC article. Review.
-
A synthetic approach toward nitiol: construction of two 1,22-dihydroxynitianes.J Org Chem. 2006 May 26;71(11):4237-45. doi: 10.1021/jo0604585. J Org Chem. 2006. PMID: 16709067
-
Schultriene and nigtetraene: two sesterterpenes characterized from pathogenetic fungi via genome mining approach.Appl Microbiol Biotechnol. 2022 Sep;106(18):6047-6057. doi: 10.1007/s00253-022-12125-4. Epub 2022 Aug 30. Appl Microbiol Biotechnol. 2022. PMID: 36040489
References
-
- Dewick P. Medicinal Natural Products: A Biosynthetic Approach. 3rd ed. Chichester, UK: John Wiley & Sons; 2009. - DOI
Publication types
LinkOut - more resources
Full Text Sources
