Real-world time trends in overall survival, treatments and patient characteristics in HR+/HER2- metastatic breast cancer: an observational study of the SONABRE Registry
- PMID: 36895447
- PMCID: PMC9989628
- DOI: 10.1016/j.lanepe.2022.100573
Real-world time trends in overall survival, treatments and patient characteristics in HR+/HER2- metastatic breast cancer: an observational study of the SONABRE Registry
Abstract
Background: This study aims to evaluate whether changes in therapeutic strategies have improved survival of patients diagnosed with hormone receptor positive (HR+), HER2 negative (HER2-) advanced breast cancer (ABC) in real-world.
Methods: All 1950 patients systemically treated for HR+/HER2- ABC and diagnosed between 2008 and 2019 in eight hospitals were retrieved from the SONABRE Registry (NCT-03577197). Patients were categorized per three-year cohorts based on year of ABC diagnosis. Tests for trend were used to examine differences in baseline characteristics, Kaplan-Meier methods and Cox proportional hazards for survival analyses, and competing-risk methods for 3-year use of systemic therapy.
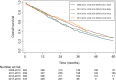
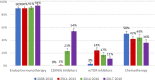
Findings: Over time, patients were older (≥70 years, 37%, n = 169/456 in 2008-2010, 47%, n = 233/493 in 2017-2019, p = 0.004) and more often had multiple metastatic sites at ABC diagnosis (48%, n = 220/456 in 2008-2010, 56%, n = 275/493 in 2017-2019, p = 0.002). Among patients with metachronous metastases the prior exposure to (neo-) adjuvant therapies increased over time (chemotherapy, 38%, n = 138/362 in 2008-2010, 48%, n = 181/376 in 2017-2019, p = <0.001; endocrine therapy, 64%, n = 231/362 in 2008-2010, 72%, n = 271/376 in 2017-2019, p = <0.001). Overall survival significantly improved from median 31.1 months (95% CI:28.2-34.3) for patients diagnosed in 2008-2010 to 38.4 months (95% CI:34.0-41.1) in 2017-2019 (adjusted hazard ratio = 0.76, 95% CI:0.64-0.90; p = 0.001). Three-year use of CDK4/6 inhibitors increased from 0% for patients diagnosed in 2008-2010 to 54% for diagnosis in 2017-2019. Conversely, three-year use of chemotherapy was 50% versus 36%, respectively.
Interpretation: Over time, patients diagnosed with HR+/HER2- ABC presented with less favourable patient characteristics. Nevertheless, we observed that overall survival of ABC increased between 2008 and 2019, with increased use of endocrine/targeted therapies.
Funding: The SONABRE Registry is supported by the Netherlands Organization for Health Research and Development (ZonMw: 80-82500-98-8003); Novartis BV; Roche; Pfizer; and Eli Lilly & Co. Funding sources had no role in the writing of the manuscript.
Keywords: CDK4/6 inhibitors; Metastatic breast cancer; Real-world; Registry; Survival.
© 2022 The Authors.
Conflict of interest statement
MM report grants institutional grants from Gilead. SG reports institutional grants from Novartis BV, Roche, Pfizer, Eli Lilly, Daiichi Sankyo and Gilead, and personal fees from Astra-Zeneca. VTH reports institutional grants and personal fees from Roche, Novartis, Pfizer, and Eli Lilly, personal fees from Accord Healthcare, institutional grants from AstraZeneca, Eisai, Daiichi Sankyo and Gilead. All remaining authors have declared no conflicts of interest.
Figures

References
-
- Gobbini E., Ezzalfani M., Dieras V., et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer. 2018;96:17–24. - PubMed
-
- Gennari A., André F., Barrios C.H., et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

